Transcranial Magnetic Stimulation (TMS) Therapy
COVID-19 Announcement
UNC is excited to announce that we are restarting our TMS service and doing so with great precautions to keep our patients and our team members healthy.
Prevention of COVID-19 infection is necessary in order to complete TMS treatment. Patients should follow recommended public health measures (social distancing, masks when social distancing is not possible, self-monitoring for symptoms) outside the clinic. If patients are symptomatic, exposed, or at high-risk, TMS may be held while they undergo evaluation and treatment for COVID and resumed after the appropriate interval. We ask that patients be unaccompanied for the duration of their clinical visits unless necessary for ambulatory assistance. Patients and providers will be asked to wear masks during the duration of the visits.
What is TMS?
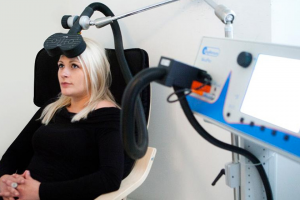 Transcranial magnetic stimulation, or TMS, is a safe, effective, and noninvasive form of brain stimulation. Approved by the US Food and Drug Administration (FDA) in 2008, TMS devices operate outside of the body and use powerful magnetic fields to stimulate nerve cells in specific areas of the brain to improve symptoms of depression and anxiety. TMS therapy is indicated for individuals diagnosed with Major Depressive Disorder (MDD) who have not achieved satisfactory symptom improvement from antidepressant medications and therapy. TMS therapy is often used in individuals who are unable to tolerate side effects from antidepressant medications such as weight gain and sexual dysfunction; however, TMS can be used alone or in conjunction with medications. TMS does not require anesthesia and patients may resume daily activities right after treatment. The UNC Outpatient psychiatry clinic currently utilizes the MagVenture TMS device with therapeutic treatments provided by a psychiatrist. Although TMS has been studied for a number of psychiatric and neurologic conditions, at this time UNC TMS clinic treats individuals who suffer from severe and/or recurrent major depressive disorder.
Transcranial magnetic stimulation, or TMS, is a safe, effective, and noninvasive form of brain stimulation. Approved by the US Food and Drug Administration (FDA) in 2008, TMS devices operate outside of the body and use powerful magnetic fields to stimulate nerve cells in specific areas of the brain to improve symptoms of depression and anxiety. TMS therapy is indicated for individuals diagnosed with Major Depressive Disorder (MDD) who have not achieved satisfactory symptom improvement from antidepressant medications and therapy. TMS therapy is often used in individuals who are unable to tolerate side effects from antidepressant medications such as weight gain and sexual dysfunction; however, TMS can be used alone or in conjunction with medications. TMS does not require anesthesia and patients may resume daily activities right after treatment. The UNC Outpatient psychiatry clinic currently utilizes the MagVenture TMS device with therapeutic treatments provided by a psychiatrist. Although TMS has been studied for a number of psychiatric and neurologic conditions, at this time UNC TMS clinic treats individuals who suffer from severe and/or recurrent major depressive disorder.
Prospective patients will meet with a TMS provider in our outpatient office for an initial consultation to discuss your psychiatric history, determine appropriateness for TMS treatment, and to learn more about our TMS clinic. The initial course of treatment typically consists of 5 treatments per week over a 4-6 week period, for an average of 25-35 total treatments. Each treatment session lasts approximately 20-40 minutes. During treatment, the patient is awake in a comfortable chair. A small magnetized wand rests lightly on the patient’s head, delivering focused magnetic stimulation directly to the area of the brain thought to be involved in regulating mood. Most insurances require a prior authorization before agreeing to pay for TMS therapy, and our offices can assist in this process.
Theta Burst Stimulation
UNC TMS Clinic is excited to announce that we offer Theta burst stimulation (TBS)! The TBS protocol was approved for use on Magventure TMS devices in August of 2018 and is a newer and faster form of TMS where the magnetic pulses are applied in a certain pattern, called bursts. Conventional TMS procedures typically last 20- 40 minutes per session whereas TBS is complete in as little as 3-5 minutes. In the “THREE-D” randomized control trial, TBS has proven to be similar to traditional TMS in regards to effectiveness, safety, and tolerability.
TMS Therapy is:
- Non-invasive
- The patient remains awake and alert during treatment and can drive themselves to and from treatment.
- Unlike vagus nerve stimulation (VNS) or deep brain stimulation (DBS), TMS does not require surgery or implantation of electrodes.
- Unlike electroconvulsive therapy (ECT), TMS does not cause seizures or require sedation with anesthesia.
- Non-systemic
- It is not taken by mouth and does not circulate in the bloodstream throughout the body.
- Safe
- Over 20 years of research in safety and effectiveness with 60+ clinical trials.
- Administered over 1 million times since FDA clearance in 2008.
- Proven
- In open-label clinical trials, 1 in 2 patients suffering from depression reported significant improvement while 1 in 3 patients were completely free of depression symptoms after six weeks of treatment.
- Targeted
- Magnetic fields do not directly affect the whole brain, but instead reach about 2-3 centimeters into the brain directly beneath the magnetic treatment coil.
- The magnetic field is specifically targeted over the left dorsolateral prefrontal cortex (DLPFC) which is the area of the brain responsible for mood regulation.
- Well-tolerated
- Individuals report few side effects from TMS and improve shortly after an individual session and decrease over time with additional sessions.
- Side effects may include headache, lightheadedness, scalp discomfort, or tingling, spasms, or twitching of facial muscles.
- TMS should not cause cognitive side effects nor does it cause weight gain, dry mouth, or upset stomach that is sometimes associated with medications.
Internal referral for interventional psych
External Interventional psych referral form
For more information, please call UNC Psychiatry TMS Clinic at (984) 974-3983.
