Prostate cancer is the most common non-skin cancer of men in the United States with over 200,000 new cases diagnosed each year. Prostate cancer can only develop in males, as the prostate gland is not present in women. The prostate is a small, walnut-shaped gland deep in the pelvis, below the bladder and in front of the rectum. It functions to produce the seminal fluid that nourishes and transports sperm for reproductive purposes.
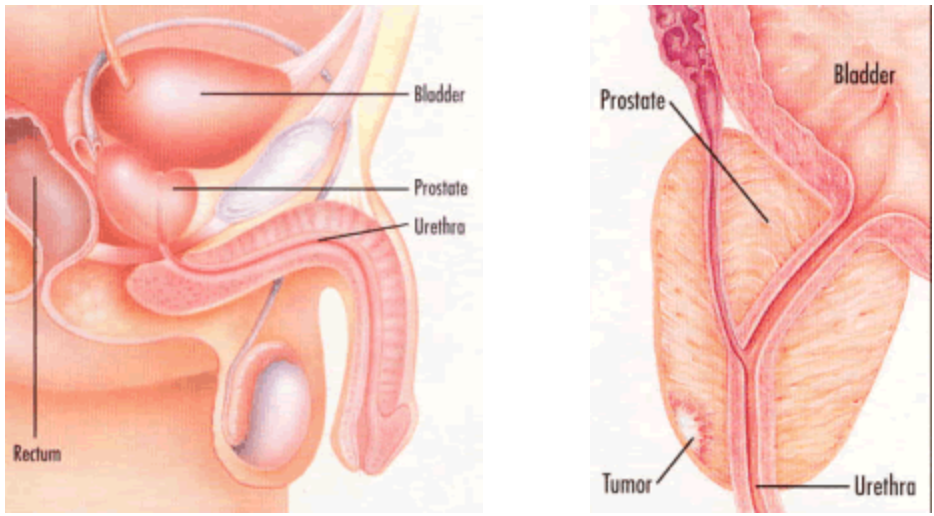
Prostate cancer begins when normal gland cells within the prostate turn into malignant or cancerous cells. As stated above, the exact mechanism of this transformation is not known and is the focus of much research performed today. A localized cluster of tumor cells can be used to grow and divide initially within the prostate itself. Cells will eventually spread beyond the outside of the prostate gland into adjacent organs (such as the seminal vesicles) and into the surrounding fatty tissue. Spread to nearby lymph nodes will occur. In advanced stages, cancer will spread to other organs – most commonly the bones, although prostate cancer is a relatively slow-growing cancer.
Risk Factors
Despite improvements in survival over the past twenty years corresponding with early detection and treatment, prostate cancer, unfortunately, remains the second most common cause of cancer deaths in US men, accounting for nearly 30,000 deaths per year.
(Read More)
Nevertheless, prostate cancer detected early—when still confined to the prostate—has a better chance of successful treatment. The lifetime risk of a US male developing prostate cancer is approximately 1 in 7, whereas about 1 man in 36 will die of prostate cancer. While prostate cancer can be a serious disease, most men diagnosed with prostate cancer do not die from it. Although the exact causes are not known, there do exist some known
risk factors for developing prostate cancer. These include the following:
- Age: Prevalence of prostate cancer increases at a near exponential rate with age; approximately 95% of cancers are diagnosed in men between the ages of 45 and 89. Roughly 6 cases out of 10 are diagnosed in men aged 65 or older (average age at diagnosis in the US is 66 years), and it is rarely diagnosed under the age of 40.
- Family history: The incidence of prostate cancers is higher in male relatives of prostate cancer patients. Although most patients have no family history, roughly 10% are at higher risk due to this factor. Persons who have one “first-degree” relative (for example a brother or a father) have a two-fold increased risk. If a person has two “first-degree” relatives, he has a 5-fold increased risk of developing prostate cancer.
- Race/Ethnicity: Prostate cancer is more common in African-American men and African-American men are also more likely to be diagnosed at an advanced stage. The risk of death from prostate cancer is more than two-fold higher in African-American men as compared to white American men. Asian-American and Hispanic/Latino men have a lower risk of prostate cancer than other groups. The reasons for these differences among racial and ethnic groups are not clear.
- Other possible risk factors: a high-fat diet, living in western countries (Western Europe, Scandinavian countries, and the United States), hormonal imbalances, and perhaps certain other dietary considerations such as vitamins (Vitamin A, Vitamin D). At the present, these are only speculated risk factors.
Symptoms
In early stages of prostate cancer, there are typically no symptoms. When—and if—the prostate becomes more extensively involved with cancer, urinary symptoms may then develop.
(Read More)
In the early stages of prostate cancer, (that is when it remains confined to the prostate), symptoms are generally not present. In fact, some of the mild urinary symptoms that patients may experience typically come from concomitant/unrelated benign enlargement of the prostate, or benign prostatic hyperplasia (BPH) and are not related to cancer that may also exist. Again, it should be emphasized that in early stages of prostate cancer, there are typically no symptoms. When—and if—the prostate becomes more extensively involved with cancer, urinary symptoms may then develop (but a very small percentage of patients have these symptoms as a consequence of their cancer). The lack of any symptoms in localized prostate cancer emphasizes the role of screening measures in patients regardless of symptoms. In most patients with prostate cancer (especially localized prostate cancer) there will usually be no symptoms. The most common symptoms of prostate cancer, though rare, can occur in advanced disease, particularly when it involves the bones. Persistent and often severe pain in the back, hips or other bones is characteristic of this stage of the disease.
Diagnosis
The initial detection of prostate cancer is made by suspicious findings on the digital rectal examination (DRE), a physical examination of the prostate gland by a healthcare provider’s gloved finger, or digit, and/or by abnormal results on a laboratory test measuring the level of prostate-specific antigen (PSA).
(Read More)
DRE and PSA are the most useful first-line tests for evaluating/screening patients for prostate cancer. The DRE consists of a rectal examination to feel for any nodules, firmness, or other abnormalities of the prostate. All men with abnormal prostate examinations—regardless of PSA levels—should undergo consultation with a urologist to determine if a prostate biopsy is indicated to rule out prostate cancer. PSA is a routine blood test for screening for prostate cancer. It is not a substitute for the rectal exam but used to complement/supplement the screening evaluation. As a single test, it is the most sensitive measure for the detection of prostate cancer. The most effective overall methods of early detection are the combined use of the digital rectal exam and the PSA.
There has been some controversy surrounding prostate cancer screening in general, and PSA testing in particular, in recent years. Patients should understand that prostate cancer screening. On the basis of a systematic literature review examining the evidence available on PSA testing, the American Urological Association made the following recommendations in 2013:
- PSA screening in men under age 40 years is not recommended.
- Routine screening in men between ages 40 to 54 years at average risk is not recommended.
- For men ages 55 to 69 years the decision to undergo PSA screening involves weighing the benefits of preventing prostate cancer mortality in 1 man for every 1,000 men screened over a decade against the known potential harms associated with screening and treatment. However, over a lifetime, this benefit could be much greater. For this reason, shared decision-making is recommended for men age 55 to 69 years that are considering PSA screening, and proceeding based on patients’ values and preferences.
- However, there are men outside this target age range (55-69 years) that could benefit from screening because they are at a higher risk of prostate cancer (race, family history, etc.). These men should discuss their risk with their physicians and assess the benefits and risks of testing
- To reduce the harms of screening, a routine screening interval of two years or more may be preferred over annual screening in those men who have participated in shared decision-making and decided on screening. As compared to annual screening, it is expected that screening intervals of two years preserve the majority of the benefits and reduce overdiagnosis and false positives.
- Routine PSA screening is not recommended for men over age 70 or any man with less than a 10-15 year life expectancy.
If an abnormality is detected on a digital rectal exam, or PSA, prostate needle biopsy should be considered in consultation with a urologist. This is commonly performed in a urologist’s office under ultrasound guidance. In order to adequately sample all the areas of the prostate, 10 to 12 biopsies—and sometimes more—are performed in that setting. It can be a slightly uncomfortable procedure, but is usually well-tolerated with local anesthesia (nerve block) and is associated with low risks of side effects (primarily bleeding and infection) when performed using standard precautionary methods. Biopsy samples are then reviewed by a pathologist and examined for the presence or absence of prostate cancer, as well as the grade and extent of cancer if it does exist.
When the diagnosis of prostate cancer is made, patients are generally assigned a grade and stage of cancer (and this is generally true for all cancer types). “Grade” refers to how aggressive cancer cells appear under the microscope, and the Gleason Score is the system used by pathologists and urologists to categorize prostate cancer cases’ grade. The “patterns” that make up the Gleason score include low-grade cancers (score 1 to 3) or higher-grade cancers (score 4 to 5). Typically, the Gleason Score will be communicated by the Pathologist as a sum, comprised of the most common pattern seen plus the next-most common pattern seen will be obtained. Low-grade cancers will typically have a sum of 2 to 6 (typically 3+3=6, but occasionally with lower numbers), intermediate grade cancers a sum of 7 (typically 3+4 or 4+3, depending upon which pattern is predominant), and high-grade cancers have a sum of 8 to 10 (typically, Gleason Scores 4+4, 4+5, 5+4, or 5+5).
The stage of cancer reflects the physical extent of cancer. Cancers confined to the prostate include stages T1 (PSA-detected, no palpable abnormality on a rectal exam) or T2 (a nodule or other abnormality palpated on a rectal exam). Both stage T1 and T2 cancers are also termed “clinically localized” disease; these are the most common stages of diagnosis in the US today. Stage T3 and T4 cancers are felt to extend outside the prostate into adjacent structures. For patients with higher-risk features (e.g., high Gleason Score and/or high PSA and/or severely abnormal prostate exam), the treating physicians will often obtain imaging tests, or scans (typically a CT scan and/or a bone scan) to test for findings that suggest cancer has spread beyond the prostate to other structures in the body. If these tests are positive, the patient may be considered stage IV, either node-positive (N+), which refers to the presence of positive lymph nodes (that is cancer involving some of the surrounding pelvic or retroperitoneal lymph nodes), and/or metastatic (M+), which refers to spread to other organs (in particular, the bones).
Staging
What are the stages/classifications of Prostate Cancer
(Read More)
- Stage I = no nodule upon exam; typically PSA detected
- Stage II = nodule confined to prostate on exam
- Stage III = spreads outside the prostate to adjacent structures = Stage T3, T4
- Stage IV = spread to lymph nodes (Stage IV, N+) and/or spread to bones or other organs (stage IV, M+)
Imaging
Learn more about the imaging techniques during the course of treatment.
(Read More)
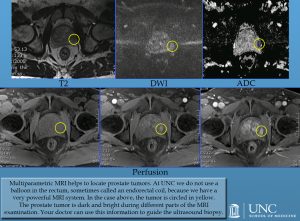
Multiparametric MRI of the prostate gland, which has been increasingly used for the last 10 years, allows the detection and localization of tumors in the prostate gland with reasonable accuracy. Additionally, multiparametric MRI may be used with reasonable accuracy to follow the patients with low-risk disease. The information acquired from MRI can also be used to guide the ultrasound biopsy.
At standard MRI systems with a magnetic field of 1.5 T, this examination is usually performed with the help of an endorectal coil, which requires the placement of a balloon in the rectum. However, at MRI systems with a higher magnetic field such as 3.0 T, this examination can be performed without the use of an endorectal coil and is performed in a similar fashion like other MRI examinations of the body including contrast administration, which helps to identify the tumor in the prostate gland. Different techniques of MRI with various accuracies for the detection and localization of tumors are used together during the prostate MRI examination in order to increase the total accuracy and therefore this technique is called as multiparametric MRI.
At UNC, we use 3.0 T MRI for multiparametric prostate MRI examinations and therefore do not use a balloon in the rectum. In the picture, the tumor is circled in yellow. The prostate tumor is dark and bright during different parts of the MRI examination. Your doctor can use this information to guide the ultrasound biopsy.
Treatment
Treatment is dependent on the stage, grade, and PSA level—which are used to assign patient to prostate cancer-specific “risk groups”—as well as patient age, health status, and personal preference, as related to the risks and benefits of various treatment options.
(Read More)
Treatments for localized cancer (stage T1, T2) include surgery (radical prostatectomy), radiation therapy (either
external beam radiation or
brachytherapy/ “seed” implant radiotherapy), or, in a significant number of cases, in particular those with lower risk features for the prostate cancer, no therapy at all (active surveillance or watchful waiting). Patients with a more advanced disease are typically treated with hormonal therapy—medications that eliminate the male sex hormone, testosterone, from the body, as prostate cancer cells typically rely on testosterone to support their growth. Again, the precise treatment strategy should be individualized for each patient after counseling with his urologist.
Surgery
Surgical removal of the prostate gland is termed a radical prostatectomy.
(Read More)
This involves removal of the gland itself, the tissue surrounding the prostate, and the adjacent seminal vesicles. In select cases, the surgeon may also remove lymph node tissue adjacent to the prostate. After the prostatectomy specimen is removed, the surgeon re-attaches the bladder to the urethra to restore continuity of the urinary tract.
Classically, the procedure can be performed through a lower abdominal incision (radical retropubic prostatectomy) or through the perineum (radical perineal prostatectomy). More recently, techniques have been developed to replicate the principles of the classic operation using minimally-invasive techniques of laparoscopic and robotic surgery. Currently, the most common approach is robotic-assisted laparoscopic prostatectomy (RALP).
Radical prostatectomy provides the highest rate of cure for prostate cancer. As an overall treatment strategy, it is perhaps best-indicated for patients under age 70 (with localized cancer) and with a greater than 10-year life expectancy. Side effects of this treatment include urinary incontinence (leakage of urine) and erectile dysfunction (impotence), due to damage to the delicate nerves responsible for erection, which run adjacent to the prostate. The specific risks of these complications are related to the surgeon’s personal experience and factors specific to the individual patient. Patients considering surgery should discuss these risks with their consulting surgeon.
The typical experience with robotic prostatectomy includes an overnight stay in the hospital, followed by a recovery period with limited activity for approximately 4 weeks. A catheter is often left across the new connection between the bladder and urethra for a period of one to two weeks.
Radiation
External Beam Radiation
(Read More)
There exists a lengthy experience, informed by a large body of clinical trials, and good long-term results with external beam radiation therapy. With this treatment, a radiation oncologist typically performs a CT scan of the pelvis to “target” radiation energy at the prostate, while trying to minimize exposure of normal surrounding tissues to potentially harmful radiation. Many centers today perform external beam radiation treatment (or EBRT) using three-dimensional “conformal” planning and/or intensity-modulated radiation therapy (IMRT), techniques that have been developed to maximize effectiveness while reducing potential harms. In some cases, patients will have “fiducial markers” or other materials placed in the prostate to help guide the delivery of the radiation therapy. Radiation therapy typically involves an extended course, including treatment 5 days a week for eight weeks. Side effects may include bladder irritation, rectal irritation, impotence, and incontinence. For patients with intermediate- or high-risk localized disease (Gleason score 7 or higher), the radiation oncologists will often recommend a course of hormonal therapy (shots to suppress the production of testosterone) temporarily; typically, before, during, and for a period of time after completion of treatment (up to three years total). Patients receiving this combined hormonal and radiation therapy may experience the side effects of hormone therapy, including hot flashes, decreased libido, breast tissue pain and/or enlargement, and fatigue.
Brachytherapy
(Read More)
Brachytherapy involves the placement of permanent radioactive seed implants into the prostate. It is typically an outpatient procedure but performed under general anesthesia. Brachytherapy appears to be as effective as external beam radiation therapy for low grade, low stage cancers, but less data is available to support its effectiveness for a higher grade and higher stage cancers.
Hormonal Therapy
(Read More)
Prostate therapy is dependent on the male sex hormone, testosterone, to grow. Therefore, by depleting the testosterone levels in patients, the prostate cancer can be put into remission. This is not considered a curative therapy, because after approximately 2 to 5 years (on average, though this is highly variable) cancer begins to grow without the necessity of testosterone, that is cancer becomes “hormone insensitive, hormone-independent”. Ways of depleting the testosterone level include surgical orchiectomy (removal of the testicles), or also can be performed via medications, which deplete the body’s testosterone levels (LHRH agonists, anti-androgens).
Watchful Waiting
(Read More)
This is essentially no therapy at all, but close surveillance. It is typically reserved for older patients (greater than 70 years old), those with poor health status, or perhaps those with lower grade, lower stage cancers and who are asymptomatic. Occasionally used for younger, healthier patients with low-grade, low-stage cancers, and only a small amount of cancers detected on biopsy. Close monitoring of the rectal exam and significant PSA changes needs to be performed, and any significant changes do warrant the intervention. It is not recommended for younger patients with long life expectancies, patients with high-grade cancers or patients with high-stage cancers. Future directions involve primarily the prevention of cancer itself. This mainly being in the form of dietary modification (for example limitation of dietary fat, vitamins, soy products, etc), medical or chemoprevention, vaccines, and perhaps some molecular and gene therapies.
Outlook
The overall incidence of prostate cancer is declining thanks to public awareness, early diagnosis, and treatment.
(Read More)
As in decades past, the majority of patients would present with metastatic disease. Today, the vast majority of patients with newly detected prostate cancer, present with clinically localized cancer that is minimal to treat and possible to cure. Clearly, much work needs to be done regarding the scientific investigation of prostate cancer, particularly in prevention, less invasive, more aggressive therapies.
Other Resources
Click here for more resources with additional information regarding Prostate Cancer.



