Children’s Research Institute Research Grant Initiative
Request for Applications
Purpose
As part of the mission of the Children’s Research Institute (CRI) to coordinate and support pediatric research, we are thrilled to support the following research funding opportunities:
- Carolina For the Kids (CFTK) Research Grant Awards
- UNC Children’s Development Early Career Investigator Grants
- IQVIA Pediatric Clinical Research Scholar Award
Please look for information on next year’s grant cycle in fall 2024.
Funding Sources and EligibilityApplicants for CRI Research Grant Initiative Awards must be a faculty member, clinical fellow or postdoctoral fellow (MD, PhD, or MD/PhD) in good standing at UNC School of Medicine or UNC Children’s Hospitals, pursuing pediatric research.
Carolina For The Kids (CFTK), a UNC student-led organization, raises funds through donations and events, including their annual dance marathon. In addition to their primary mission of providing emotional, medical, and financial support for the patients and families served by UNC Children’s Hospital, CFTK supports a Research Grant Program. This program prioritizes clinical/translational research projects focused on children’s health. This mechanism supports early career and established faculty investigators (CFTK Faculty Investigator Award), as well as clinical and postdoctoral fellows (CFTK Fellows Award), who have obtained a MD, PhD, or MD/PhD. Projects primarily or wholly based at UNC may be prioritized, at the discretion of CFTK’s executive leadership committee.
CFTK Faculty Investigator Award
- Projected Maximum Award Amount: $40,000.
- Eligibility: Applicant must have obtained a MD, PhD, or MD/PhD, and be one of the following:
- Early career faculty investigators who have not previously competed successfully for an NIH-supported research project, specifically Assistant Professors or Instructors who are generating preliminary data for an extramural grant application.
- Established faculty investigators with a track record of NIH funding and scholarly productivity in need of bridge funding (investigator salary support is not covered). Investigators who have submitted an NIH grant that received positive reviews but was not funded are eligible. The application must include the review summary statement, responses to noted weaknesses, and plan for resubmission.
- Established faculty investigators who are expanding or redirecting research activities toward a new extramural funding opportunity.
- Requirements: Applications must present a clear research proposal and a plan for subsequent extramural funding. It is expected that an extramural grant application will be submitted within 6 months of the completion of the CFTK Research proposal.
CFTK Fellows Award
- Project Maximum Award Amount: $5,000.
- Eligibility: Fellows in a pediatric training program performing research with a child health focus or postdoctoral research fellows performing research with a child health focus (MD, PhD, MD/PhD).
- Requirements: Applicants must identify a mentor who is committed to project and financial oversight and will work closely with the applicant on project design, execution and presentation/publication. The application must include a mentor’s letter of support (which articulates the mentor’s engagement with the applicant and project) and biosketch. Faculty mentors partnering with fellows will assume responsibility for stewardship of funds and program compliance, including any issues following the fellow’s graduation or departure from the program. Signature documentation of this agreement is required and is part of the application.
This grant is funded by the UNC Children’s Health Foundation through fundraising and philanthropic donations. Oversight comes from the Department of Pediatrics Chair, Dr. Stephanie Davis. This grant supports the development of pediatric researchers to advance child health. In alignment with this mission, this mechanism supports early career investigators who perform pediatric-related research in basic, translational, or clinical research, domestically or abroad.
- Project Maximum Award Amount: $10,000.
- Eligibility: Early career faculty at the rank of Assistant Professor performing pediatric research (MD, PhD, or MD/PhD).
- Requirements: This award is intended to support a research project spearheaded by an early career investigator working with guidance of an identified senior faculty mentor. Proposed projects can include ancillary projects to already established or planned (funded) research protocols, stand-alone research projects, or projects with a quality improvement focus (as long as special consideration is given towards plans for publication). The application must include the mentor’s letter of support (which articulates the mentor’s engagement with the mentee and project) and biosketch. Plans and considerations for leveraging the products of the proposed work for the pursuit of future extramural funding are highly encouraged.
IQVIA is a worldwide leader in data science, providing access to real-world data, advanced analytics, innovative technologies, and healthcare expertise. IQVIA uses its unique expertise to drive new healthcare discoveries, support more informed decision-making, and make possible new opportunities in medical research of vulnerable populations. IQVIA’s strength in data science has led to the generation and dissemination of real-world evidence by enabling academic researchers, like those at the CRI, to garner knowledge from existing datasets, which can lead to research findings that drive clinical practice. IQVIA’s unique strengths in healthcare data science catalyze innovation in healthcare, identifying proper treatments for patients, improving access and delivery of healthcare, and ultimately driving better health outcomes.
- Award amount: Up to $50,000 per year for up to 3 years, contingent upon progress as determined by review of required annual progress reports by IQVIA and the CRI Review Committee.
- Eligibility: Early Career Investigators at the rank of Assistant or Associate Professor within the UNC Department of Pediatrics are invited to apply.
- Requirements: IQVIA is partnering with the CRI to support the development of the next generation of physician-scientists at UNC. The IQVIA Pediatric Clinical Research Scholar is meant to support the research and career development of an early-stage investigator with a focus on data science, children’s health, and the development of new treatments. Funding from this award may support activities such as, but not limited to, the generation of preliminary data for future research proposals, pilot/feasibility studies, ancillary investigations to ongoing clinical research/trials, and career development opportunities.
FAQs specific to this opportunity (overriding other guidance on this page):
- Salary/fringe support for the awardee is allowed.
- Costs related to professional development/training (coursework/workshop participation/etc.) are allowed.
- A description of how the opportunity will also advance clinical research training of additional early-stage investigators (i.e. fellows/residents), should be included when applicable.
- Use of IQVIA resources is not a requirement. If you are interested in exploring the resources available through IQVIA, please Jinhee Lee (jinheel@email.unc.edu) to arrange a consult.
- Award selection is handled jointly by IQVIA and CRI leadership and does not necessarily follow the review process outlined generally on this page.
- Annual progress reports include a formal written report and video conferencing with IQVIA staff members.
The CRI requires that potential applicants inform us of their intention to submit by May 20th at 5 pm EST at childrensresearch@med.unc.edu (please include your proposal’s working title and a draft abstract). Final proposals will be due by June 3rd, 2024 at 5 pm EST.
The Review Committee
The Review Committee is comprised of Department of Pediatrics, UNC Children’s Hospital, and School of Medicine faculty members, representing diversity in research experience and demographics. Members serve on a rotating basis. Final funding decisions are made in conjunction with the funders.
Written Proposal Review Process
Each proposal is assigned to a primary and secondary reviewer whose research aligns with the proposed study whenever possible. Reviewers are asked to review the specific aims for all proposal submissions and required to review the entire application packet for only those they are assigned as primary or secondary reviewer. Reviewers will use the NIH-based criteria below to evaluate and generate an overall score (see Scoring Scale below), for the proposal. For applicants to the Carolina for the Kids Fellow Award, initial reviewer scores will be used in deciding who to invite for a presentation.
Review Criteria:
- Significance: What is the problem/barrier the proposal seeks to address? If the aims or the proposal are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved and how will this advance the overall field?
- Approach: Is the hypothesis valid (is the rationale and any supporting evidence sound)? Is the overall strategy, methodology, and/or proposed analyses well-reasoned and appropriate to address the specific aims? Are the specific aims well supported by the rationale and evidence provided? Are they generally robust and feasible to achieve given the project’s timeline and resources? Are potential problems or alternative strategies appropriately discussed and addressed? What are the overall strengths and weaknesses of the proposal in terms of approach?
- Innovation: Does the proposal challenge/propose refinements to current clinical, research, or theoretical paradigms? Does the proposal utilize novel concepts, approaches/methods, instrumentation, or interventions? What are the overall strengths and weaknesses of the innovative aspects of the proposal?
- Investigator: For established investigators, how does their experience, skills, and record lend themselves to what is proposed? For early career faculty, does the mentor or identified collaborative team meet qualifications in regards to accomplishing the goals of this proposal? Are the investigator and their team well suited to successfully carry out the goals of the proposal?
- Extramural Funding: If applicable, are plans for future extramural funding applications appropriate? If intended to support a previously unfunded NIH grant submission, would this proposal significantly address the major critiques or lack of preliminary data highlighted in the reviews of that proposal?
- Appropriate budget: Does it conform to the regulations of the funding announcement? Is it reasonable in light of the described approach and any additional resources available to the investigator(s)?
- For current or prior CRI grant recipients, conforming to the requirements of past awards.
In addition, research projects involving human subjects will:
- Provide sample size driven by biostatical-supported power calculations, or rationale as to why biostatistical calculations do not apply.
- Demonstrate feasibility, including:
- Study population (e.g., commitment of clinics involved for potential recruitment, population numbers from i2b2 or Carolina Data Warehouse)
- Resources for implementation, (e.g., clinic availability, coordinator support)
- Plan for human subjects/IRB approval
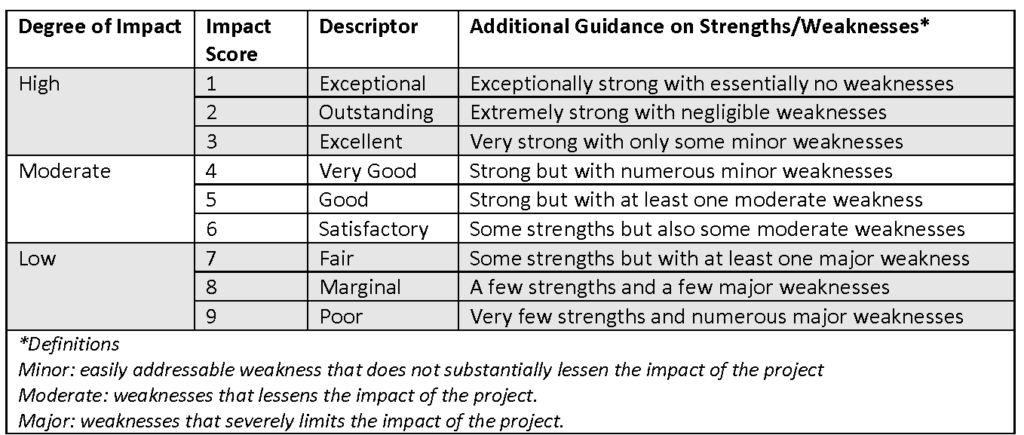
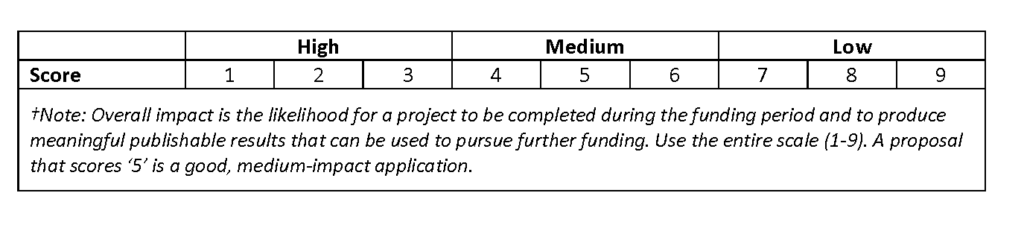
For each proposal, reviewers will score and provide comments and recommendations on areas for improvement on its Significance, Approach, Innovation and Investigator (please see “Scoring Guide,” in Scoring Scale below). In addition, reviewers will comment on the project’s potential for extramural funding and its budget, as well as to provide an Overall Impact Score for the proposal (please see “Overall Impact Guide,” in Scoring Scale below).
Scoring Scale
Reviewers will use the scale below to assign scores:
Reviewers who score 3 or more points apart from each other for the same application will be alerted to the discrepancy and asked to deliberate the discrepancy.
Oral Presentations (Clinical Fellow/Postdoctoral Fellow Applications Only)
- The highest scoring written proposal applicants will be invited to give an oral presentation. Based upon relative scores of the reviews, proposals with impact scores of 7-9 will not be considered in the current year.
- All reviewers attend and listen to each presentation.
- All reviewers are asked to read the proposal application, paying attention to the specific aims page, of every invited presenter.
- Mentors may attend presentations given by fellows.
- Each presenter will have 5 minutes for their talk followed by 5 minutes for reviewer questions.
- After the presenter leaves the room, reviewers have 10 minutes to discuss the presentation and application, then submit a score based on the presentation and application online (see Scoring Scale above).
- Primary and secondary reviewers will summarize the proposal and then the entire committee with briefly discuss.
- After all presentations are given and scored, scores will be ranked within the funding category and the Review Committee will recommend each grant be either “Recommended for Funding” or “Not Recommended for Funding” based on the ranking.
- In cases where there is a tie, primary and secondary reviewers of the proposals will speak to the strengths and weaknesses for consideration
- Top proposals are then submitted to the Carolina for the Kids Executive Directors for final approval.
- Proposals requiring revisions are still submitted to CFTK for approval, with funding contingent upon revisions received and approved by CRI.
Feedback from the Review Committee
All applicants will receive review scores and comments from their application back from the primary and secondary reviewers, alongside additional notes from the oral presentation if applicable. Those applicants selected and approved for funding should receive final notice of award within 6 months from submission.
Post Award Process & Financial Management
Following the notice of award, a specific start date will be established. Timelines for the funding period are set by each individual funding opportunity.
- Carolina for the Kids Faculty and Fellow Research Grants: 18 months
- UNC Children’s Development Early Career Investigator Grants: 18 months
- IQVIA Pediatric Clinical Research Scholar Award: 12 months, renewable for up to 3 years
PIs will have from the date of award to complete their research, including time to receive regulatory and other approvals. Investigators will not have access to the funds until all appropriate University, IACUC, or IRB approvals have been received and the appropriate accounts are created by the UNC Department of Pediatrics financial data systems. Any proposal revisions that are requested by the review committee must be accepted before funds will be disbursed.
PIs are responsible for maintaining the necessary approvals and maintaining current certifications of training in research (IRB/OHRP, IAUCUC, EHS, HIPAA, etc.). For all grants, one extension of 12 months may be allowed following the original grant period. Extensions require approval of the CRI. All requests for extensions will require investigators to update CRI on their progress and timeline. Failure to do so will result in extension request being denied, with remaining funds returning to CRI. The approval process may also include meetings between the CRI’s grant team, the investigator and division leaders as needed to identify roadblocks in research and discuss possible solutions. Following the conclusion of the extension period, a financial review of the account will be performed. Accounts will be left open until any open or incurred balances are cleared, with any leftover balance of funds returning to CRI.
If an investigator receives an award between the date of submission and the date of review for a project similar in type and scope, this must be brought to the attention of the CRI Grant Initiative team immediately. A summary of the newly funded project, areas of overlap, and a proposal for a revised budget, if requested, should be emailed directly to the CRI team at childrensresearch@med.unc.edu. Departmental and CRI leadership will review and make determinations for potential modifications to the award.
Award recipients will be expected to present updates on their research project and its findings at a future occurrence of the CRI Seminar Series.
Research Grant Account Reviews
Account reviews are mandatory, both after a time period of 12 months, and at completion of the project. Any funds remaining in the project after the end of the funding period will be returned to the Grants program. If an extension is granted, additional account reviews will be required.
Continued funding is contingent upon progress as determined from the recipient’s annual progress report and CRI Review Committee review and approval. If an account incurs a negative balance in the course of the award period, the outstanding amount will be the responsibility of the awardee, to be addressed through consultation with awardee’s division administrator and chief.
Grant Tracking Program
Awardees will be required to complete brief progress reports after 12 months of funding and a final progress report to track milestones and progress of the research projects. The CRI will request updates on these plans and the success of any funding applications via a brief survey. Problems severely hampering the progress of the projects should be brought to the attention of the CRI as soon as possible, who will work with the awardees to try to address these issues. Failure to complete progress reports in a timely manner may result in early termination of a project and forfeiture of remaining funds.
All Research Grant awards will be entered into a tracking program to evaluate the productivity of investigators related to extramural funding applications, awards, publications and development. Grant and manuscript tracking of award productivity begins on the date of the award (account set-up date). Future Research Grant applications will have this tracking information made available to the reviewers and the Research Grants committee for consideration in their deliberations. Investigators that have successfully fulfilled the grant submission and publication requirements within the appropriate period of time will have this accomplishment highlighted during the application process for future Research Grant funding.
Citing the Research Grants
Carolina for the Kids Research Grant-supported researchers will acknowledge support from the Carolina for the Kids Research Grants program in their publications and presentations (e.g., “This research was supported, in part, by the Carolina For The Kids Grants Program.”)
UNC Children’s Development Early Career Researcher Grants will acknowledge support from the UNC Health Foundation in their publications and presentations (e.g., “This research was supported, in part, by a UNC Children’s Development Early Career Investigator grant through the generous support of donors to UNC.”)
IQVIA Scholars Award supported researchers will acknowledge support from IQVIA in their publications and presentations (e.g., “This research was supported, in part, by an IQVIA Scholars Award.”)
Please look for information on next year’s cycle in fall 2024.
The online application for all CRI Grant Initiative Awards can be found here. You will need to create an account with Foundant and submit your application online. You will select which CRI Grant Initiative Award you are applying for within the Foundant system. Each applicant may submit up to one proposal per funding mechanism per year. The system will permit one application per submission.
You will be asked to complete information to identify you, your research, and department. You will also be asked if your application underwent a design and statistical review prior to the submission deadline date, or an explanation why this was not performed. This may be performed by a fellow investigator, mentor, collaborator or biostatistician. Please be sure the study’s stated aims and the statistical analysis plans are consistent.
You will also be asked to upload:
Proposal Documents
Please upload in pdf format, using 0.5’’ margins, Arial font-11 point or larger. There are no restrictions on font color, although black/high-contrast colors are recommended. Font size for legends/figures should be no smaller than 9 point. Letters of Support do not need to conform to these formatting restrictions.
- Abstract (1/2 page)
- Specific Aims (1 page)
- Project description (Limited to 2 pages; an additional third page can be utilized for figures or tables as needed)
- Impact on career (faculty) or training (fellow) (1 page limit)
- Timetable (1 page-describe the time from award to completion of milestones such as IRB approval, first patient enrolled, rate of anticipated patient enrollment, completion of specimen analysis or data acquisition, start and end of interim/final analyses)
- Applicant Biosketch in NIH format
- Faculty mentor Biosketch in NIH format (Fellows and Early Career Investigators only)
- Faculty Mentor Letter of Support (Fellows and Early Career Investigators only, see Additional Instructions below)
- Division Chief Letter of Support (See additional instructions below)
- Additional Letters of Support as needed from potential collaborators
- Plans for submission/resubmission of proposal(s) for extramural funding resultant from the proposed work (1/2 page)
- Budget (please download and use this budget template for your proposal)
- Budget Justification
- For Proposals that will support a resubmission of a favorably reviewed, but unfunded NIH application, applicants should provide the NIH review summary statement and submitted responses to noted weaknesses returned to the reviewers
Budget and Budget Justification
Many expenses may not be covered. Note: These lists are not necessarily all-inclusive. Please contact the Children’s Research Institute at childrensresearch@med.unc.edu with any questions about whether or not the research funds cover any possible expense.
Approved Research Expenses:
- Biostatistical support
- Computer/software (with clear justification of why they are needed). Any information technology-related purchases deemed essential to the project by the awards committee must be approved by Department of Pediatrics IT. All hardware and software will be considered property of the Department. The Department will determine appropriate disposition of such items at the end of the project.
- Equipment and supplies
- Office supplies
- Postage (for research-related mailings)
- Publication costs of publishers (submit info from publisher)
- Salary and fringe benefit (UNC faculty not allowed; other research positions may be allowed, but must be approved by PI’s department via the routing/signature process.)
- Travel
Not Approved Expenses:
- Any expense not directly related to research activity
- Any personal professional expense
- Board exam fees
- Board review courses
- DEA#
- Grant writing/editing for submissions, articles, etc.
- Lab coats
- Medical licenses
- Membership dues to societies and professional organizations
- Periodicals
- Professional liability insurance
- Subscriptions
- Telephone and pager
Faculty Mentor Letter of Support (for mentored awards)
Assistant Professors and Fellows must apply with a faculty mentor. Faculty mentors partnering with fellows or early career investigators will assume responsibility for stewardship of funds and program compliance, including any issues remaining following the applicant’s graduation and departure from the University. Include in the Faculty Mentor Letter of Support:
- Mentor’s support and approval of the proposed research plan and commitment toward actively mentoring the applicant.
- Mentor’s agreement to fund stewardship and program compliance as described above.
- Mentor’s prior experience in mentoring trainees.
- Assurance of adequate time available from the mentor needed to oversee the project.
- Assurance that the applicant has access to adequate resources and/or equipment required to carry out the proposed work (for items not requested in the budget).
- A commitment by the mentor to help the fellow present or publish the research.
- An agreement by the mentor to ensure compliance with the reporting requirements described in Post Award Expectations and Responsibilities.
Division Chief Letter of Support
All applicants must provide a letter of support from their division chief. Include in the Division Chief Letter of Support:
- Assurance that the applicant has adequate time and support to complete the project within the grant period.
- Assurance that the applicant has access to adequate resources and/or equipment required to carry out the proposed work.
I am currently a fellow, but will be an Assistant Professor by the time the award period starts. Am I eligible to apply for awards given only to Assistant Professors?
Yes. You should include an additional letter of support from your division chief or department chair that includes the position title and start date of your appointment.
Is salary support allowed for Carolina for the Kids and Early Investigator awards?
Salary support for Principal Investigators and Co-Investigators is not allowed; however, salary support for research staff (research associates, technicians, biostatisticians) is allowed.
My primary appointment is not in the Department of Pediatrics. Can I still apply?
Yes. These awards are designed to support research occurring within the Department of Pediatrics and at UNC Children’s. Applicants must be affiliated with either the Department of Pediatrics or UNC Children’s.
Are reference pages/bibliographies included in the page limits for each section of the application?
No. Your bibliography page does not count towards page limits.
Is a letter of intent required?
Letters of intent are encouraged, but not required. They can be emailed to childrensresearch@med.unc.edu. Letters of intent should include name of applicant, their proposed topic/research area and, for early career investigators, the name of their mentor(s).
What if my funding changes between the time I submit the grant and when I am awarded?
Any awards of similar project type and scope received between the submission date and review date must be brought to the attention of CRI Grant Initiative Team. Updated budgets can be emailed directly to childrensresearch@med.unc.edu.