Specialty Areas
Airway Epithelial Stem Cells, Airway Innate Immunity and Response to Injury, mRNA regulation
Research Focus
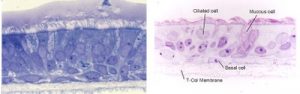
Identification of airway epithelial stem cells. Dr. Randell’s goals are to identify and isolate airway epithelial stem cells and to understand molecular mechanisms regulating airway epithelial cell proliferation and fate decisions.Research in Dr. Randell’s laboratory is currently focused on five areas where a greater knowledge of basic cell biology can be applied towards overcoming clinical lung disease problems:
- Micro RNA Regulation of Human Airway Epithelial Phenotype- ARRA Award. The Randell Laboratory comprehensively determines the miRNA repertoire of human airway epithelial cells will tests the ability of miRNAs to alter cell structure and function. These studies will create a valuable database and will suggest novel treatments for lung disease.
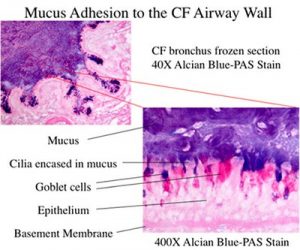
- Innate immunity in the airway. Dr Randell aims to better understand molecular mechanisms regulating airway epithelial adaptation to injury and chronic infection and to understand the relationship of adaptation to the pathogenesis of airway disease.
- Post-lung transplant ischemia reperfusion injury and bronchiolitis obliterans syndrome. The Randell Laboratory is also examining basic mechanisms of lung injury relevant to transplantation and bronchiolitis obliterans syndrome, the leading cause of lung allograft failure.
- Dr. Scott Randell also directs the UNC Marsico Lung Institute (MLI) Tissue Procurement and Cell Culture Core, which provides primary and passaged human airway and alveolar epithelial cells, lung microvascular endothelial cells, media and expertise to UNC MLI investigators and collaborators. Our facility has become a nationally and internationally recognized resource whose services are sought for collaboration, contract research, and training by academics, non-profit organizations, biotech and the pharmaceutical industry.
Staff

Teresa Mascenik
Research Specialist

Tuong Nguyen
Research Technician

Rhianna Lee
Research Collaborator
Selected Bibliography
- Lee RE, Mascenik TM, Major SC, Galiger JR, Bulik-Sullivan E, Siesser PF, Lewis CA, Bear JE, Le Suer JA, Hawkins FJ, Pickles RJ, Randell SH. Viral Airway Injury Promotes Cell Engraftment in an In Vitro Model of Cystic Fibrosis Cell Therapy. Am J Physiol Lung Cell Mol Physiol. 2024 Mar 1;326(3):L226-L238. doi: 10.1152/ajplung.00421.2022. PMID: 38150545.
- Asakura T, Okuda K, Chen G, Dang H, Kato T, Mikami Y, Schworer SA, Gilmore RC, Radicioni G, Hawkins P, Barbosa Cardenas SM, Saito M, Cawley AM, De la Cruz G, Chua M, Alexis NE, Masugi Y, Noone PG, Ribeiro CMP, Kesimer M, Olivier KN, Hasegawa N, Randell SH, O’Neal WK, Boucher RC. Proximal and Distal Bronchioles Contribute to the Pathogenesis of Non-Cystic Fibrosis Bronchiectasis (NCFB). Am J Respir Crit Care Med. 2024 Feb 15;209(4):374-389. doi: 10.1164/rccm.202306-1093OC. PMID: 38016030. PMCID: PMC10878387.
- Hou YJ, Chiba S, Leist SR, Meganck RM, Martinez DR, Schäfer A, Catanzaro NJ, Sontake V, West A, Edwards CE, Yount B, Lee RE, Gallant SC, Zost SJ, Powers J, Adams L, Kong EF, Mattocks M, Tata A, Randell SH, Tata PR, Halfmann P, Crowe JE Jr, Kawaoka Y, Baric RS. Host range, transmissibility and antigenicity of a pangolin coronavirus. Nat Microbiol. 2023 Oct;8(10):1820-1833. doi: 10.1038/s41564-023-01476-x. PMID: 37749254; PMCID: PMC10522490.
- Tse LV, Hou YJ, McFadden E, Lee RE, Scobey TD, Leist SR, Martinez DR, Meganck RM, Schäfer A, Yount BL, Mascenik T, Powers JM, Randell SH, Zhang Y, Wang L, Mascola J, McLellan JS, Baric RS. A MERS-CoV antibody neutralizes a pre-emerging group 2c bat coronavirus. Sci Transl Med. 2023 Sep 27;15(715):eadg5567. doi: 10.1126/scitranslmed.adg5567. PMID: 37756379.
- Ma L, Thapa BR, Le Suer JA, Tilston-Lünel A, Herriges MJ, Berical A, Beermann ML, Wang F, Bawa PS, Kohn A, Ysasi AB, Kiyokawa H, Matte TM, Randell SH, Varelas X, Hawkins FJ, Kotton DN. Airway stem cell reconstitution by the transplantation of primary or pluripotent stem cell-derived basal cells. Cell Stem Cell. 2023 Sep 7;30(9):1199-1216.e7. doi: 10.1016/j.stem.2023.07.014. PMID: 37625411. PMCID: PMC10528754.
- Lee RE, Reidel B, Nelson MR, Macdonald JK, Kesimer M, Randell SH. Air-Liquid interface cultures to model drug delivery through the mucociliary epithelial barrier. Adv Drug Deliv Rev. 2023 Jul;198:114866. doi: 10.1016/j.addr.2023.114866. PMID: 37196698. PMCID: PMC10336980.
- Mikami Y, Grubb BR, Rogers TD, Dang H, Asakura T, Kota P, Gilmore RC, Okuda K, Morton LC, Sun L, Chen G, Wykoff JA, Ehre C, Vilar J, van Heusden C, Livraghi-Butrico A, Gentzsch M, Button B, Stutts MJ, Randell SH, O’Neal WK, Boucher RC. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023 Jun 7;15(699):eabo7728. doi: 10.1126/scitranslmed.abo7728. PMID: 37285404. PMCID: PMC10664029.
- Tyrrell J, Ghosh A, Manzo ND, Randell SH, Tarran R. Evaluation of chronic cigarette smoke exposure in human bronchial epithelial cultures. J Appl Toxicol. 2023 Jun;43(6):862-873. doi: 10.1002/jat.4430. PMID: 36594405.
- Rustam S, Hu Y, Mahjour SB, Rendeiro AF, Ravichandran H, Urso A, D’Ovidio F, Martinez FJ, Altorki NK, Richmond B, Polosukhin V, Kropski JA, Blackwell TS, Randell SH, Elemento O, Shaykhiev R. A Unique Cellular Organization of Human Distal Airways and Its Disarray in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2023 May 1;207(9):1171-1182. doi: 10.1164/rccm.202207-1384OC. PMID: 36796082. PMCID: PMC10161760.
- Barnett KC, Xie Y, Asakura T, Song D, Liang K, Taft-Benz SA, Guo H, Yang S, Okuda K, Gilmore RC, Loome JF, Oguin III TH, Sempowski GD, Randell SH, Heise MT, Leo Lei Y†, Boucher RC†, Ting JPY†. An epithelial-immune circuit amplifies inflammasome and IL-6 responses to SARS-CoV-2. Cell Host Microbe. 2023 Feb 8;31(2):243-259.e6. doi: 10.1016/j.chom.2022.12.005. PMID: 36563691. PMCID: PMC9731922. †These authors equally supervised this work.
- Kato T, Asakura T, Edwards CE, Dang H, Mikami Y, Okuda K, Chen G, Sun L, Gilmore RC, Hawkins P, De la Cruz G, Cooley MR, Bailey AB, Hewitt SM, Chertow DS, Borczuk AC, Salvatore S, Martinez FJ, Thorne LB, Askin FB, Ehre C, Randell SH, O’Neal WK, Baric RS, Boucher RC; NIH COVID-19 Autopsy Consortium. Prevalence and mechanisms of mucus accumulation in COVID-19 lung disease. Am J Respir Crit Care Med. 2022 Dec 1;206(11):1336-1352. doi: 10.1164/rccm.202111-2606OC. PMID: 35816430. PMCID: PMC9746856.
- Kim J, Rustam S, Mosquera JM, Randell SH, Shaykhiev R, Rendeiro AF, Elemento O. Unsupervised discovery of tissue architecture in multiplexed imaging. Nat Methods. 2022 Dec;19(12):1653-1661. doi: 10.1038/s41592-022-01657-2. PMID: 36316562.
- Lee RE, Lewis CA, He L, Bulik-Sullivan EC, Gallant SC, Mascenik TM, Dang H, Cholon DM, Gentzsch M, Morton LC, Minges JT, Theile JW, Castle NA, Knowles MR, Kimple AJ, Randell SH. Small-molecule eRF3a degraders rescue CFTR nonsense mutations by promoting premature termination codon readthrough. J Clin Invest. 2022 Sep 15;132(18):e154571. doi: 10.1172/JCI154571. PMID: 35900863; PMCID: PMC9479597.
- Esther CR Jr, Kimura KS, Mikami Y, Edwards CE, Das SR, Freeman MH, Strickland BA, Brown HM, Wessinger BC, Gupta VC, Von Wahlde K, Sheng Q, Huang LC, Bacon DR, Kimple AJ, Ceppe AS, Kato T, Pickles RJ, Randell SH, Baric RS, Turner JH, Boucher RC. Pharmacokinetic-based failure of a detergent virucidal for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) nasal infections: A preclinical study and randomized controlled trial. Int Forum Allergy Rhinol. 2022 Sep;12(9):1137-1147. doi: 10.1002/alr.22975. PMID: 35040594. PMCID: PMC9011886.
- Berical A, Lee RE, Lu J, Beermann ML, Le Suer JA, Mithal A, Thomas D, Ranallo N, Peasley M, Stuffer A, Bukis K, Seymour R, Harrington J, Coote K, Valley H, Hurley K, McNally P, Mostoslavsky G, Mahoney J, Randell SH, Hawkins FJ. A multimodal iPSC platform for cystic fibrosis drug testing. Nat Commun. 2022 Jul 29;13(1):4270. doi: 10.1038/s41467-022-31854-8. PMID: 35906215; PMCID: PMC9338271.
- Hamad SH, Montgomery SA, Simon JM, Bowman BM, Spainhower KB, Murphy RM, Knudsen ES, Fenton SE, Randell SH, Holt JR, Hayes DN, Witkiewicz AK, Oliver TG, Major MB, Weissman BE. TP53, CDKN2A/P16, and NFE2L2/NRF2 regulate the incidence of pure- and combined-small cell lung cancer in mice. Oncogene. 2022 Jun;41(25):3423-3432. doi: 10.1038/s41388-022-02348-0. PMID: 35577980.
- Kadur Lakshminarasimha Murthy P, Sontake V, Tata A, Kobayashi Y, Macadlo L, Okuda K, Conchola AS, Nakano S, Gregory S, Miller LA, Spence JR, Engelhardt JF, Boucher RC, Rock JR, Randell SH, Tata PR. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature. 2022 Apr;604(7904):111-119. doi: 10.1038/s41586-022-04541-3. PMID: 35355018. PMCID: PMC9169066.
- Kato T, Radicioni G, Papanikolas MJ, Stoychev GV, Markovetz MR, Aoki K, Porterfield M, Okuda K, Barbosa Cardenas SM, Gilmore RC, Morrison CB, Ehre C, Burns KA, White KK, Brennan TA, Goodell HP, Thacker H, Loznev HT, Forsberg LJ, Nagase T, Rubinstein M, Randell SH, Tiemeyer M, Hill DB, Kesimer M, O’Neal WK, Ballard ST, Freeman R, Button B, Boucher RC. Mucus concentration-dependent biophysical abnormalities unify submucosal gland and superficial airway dysfunction in cystic fibrosis. Sci Adv. 2022 Apr;8(13):eabm9718. doi: 10.1126/sciadv.abm9718. PMID: 35363522.
- Vaidyanathan S, Baik R, Chen L, Bravo DT, Suarez CJ, Abazari SM, Salahudeen AA, Dudek AM, Teran CA, Davis TH, Lee CM, Bao G, Randell SH, Artandi SE, Wine JJ, Kuo CJ, Desai TJ, Nayak JV, Sellers ZM, Porteus MH. Targeted replacement of full-length CFTR in human airway stem cells by CRISPR-Cas9 for pan-mutation correction in the endogenous locus. Mol Ther. 2022 Jan 5;30(1):223-237. doi: 10.1016/j.ymthe.2021.03.023. PMID: 33794364. PMCID: PMC8753290.
- Kabadi AM, Machlin L, Dalal N, Lee RE, McDowell I, Shah NN, Drowley L, Randell SH, Reddy TE. Epigenome editing of the CFTR-locus for treatment of cystic fibrosis. J Cyst Fibros. 2022 Jan;21(1):164-171. doi: 10.1016/j.jcf.2021.04.008. PMID: 34049825; PMCID: PMC8613331.
- Kong X, Bennett WC, Jania CM, Chason KD, German Z, Adouli J, Budney SD, Oby BT, van Heusden C, Lazarowski ER, Jaspers I, Randell SH, Hedgespeth BA, Cruse G, Hua X, Schworer SA, Smith GJ, Kelada SN, Tilley SL. Identification of an ATP/P2X7/mast cell pathway mediating ozone-induced bronchial hyperresponsiveness. JCI Insight. 2021 Nov 8;6(21):e140207. doi: 10.1172/jci.insight.140207. PMID: 34546976; PMCID: PMC8663556.
- Sims AC, Mitchell HD, Gralinski LE, Kyle JE, Burnum-Johnson KE, Lam M, Fulcher ML, West A, Smith RD, Randell SH, Metz TO, Sheahan TP, Waters KM, Baric RS. Unfolded Protein Response Inhibition Reduces Middle East Respiratory Syndrome Coronavirus-Induced Acute Lung Injury. mBio. 2021 Aug 31;12(4):e0157221. doi: 10.1128/mBio.01572-21. PMID: 34372702; PMCID: PMC8406233.
- Okuda K, Randell SH, Birket SE. The Big Impact of Small Airway pH. Am J Respir Cell Mol Biol. 2021 Aug;65(2):123-125. doi: 10.1165/rcmb.2021-0070ED. PMID: 33831321; PMCID: PMC8399579.
- Marozkina N, Smith L, Zhao Y, Zein J, Chmiel JF, Kim J, Kiselar J, Davis MD, Cunningham RS, Randell SH, Gaston B. Somatic cell hemoglobin modulates nitrogen oxide metabolism in the human airway epithelium. Sci Rep. 2021 Jul 29;11(1):15498. doi: 10.1038/s41598-021-94782-5. PMID: 34326365; PMCID: PMC8322277.
- Okuda K, Randell SH, Birket SE. The Big Impact of Small Airway pH. Am J Respir Cell Mol Biol. 2021 Jul 29;11(1):15498. doi: 10.1165/rcmb.2021-0070ED. PMID: 33831321. PMCID: PMC8399579.
- Benway CJ, Liu J, Guo F, Du F, Randell SH, Cho MH, Silverman EK, Zhou X; International COPD Genetics Consortium. Chromatin Landscapes of Human Lung Cells Predict Potentially Functional COPD GWAS Variants. Am J Respir Cell Mol Biol. 2021 Jul;65(1):92-102. doi: 10.1165/rcmb.2020-0475OC. PMID: 33788674. PMCID: PMC8320120.
- Dang Y, van Heusden C, Nickerson V, Chung F, Wang Y, Quinney NL, Gentzsch M, Randell SH, Moulton HM, Kole R, Ni A, Juliano RL, Kreda SM. Enhanced delivery of peptide-morpholino oligonucleotides with a small molecule to correct splicing defects in the lung. Nucleic Acids Res. 2021 Jun 21;49(11):6100-6113. doi: 10.1093/nar/gkab488. PMID: 34107015; PMCID: PMC8216463.
- Kato T, Mikami Y, Sun L, Rogers TD, Grubb BR, Morrison CB, Ehre C, Sears PR, Ostrowski LE, Randell SH, Boucher RC. Reuse of Cell Culture Inserts for in vitro Human Primary Airway Epithelial Cell Studies. Am J Respir Cell Mol Biol. 2021 Jun;64(6):760-764. doi: 10.1165/rcmb.2021-0033LE. PMID: 33788673. PMCID: PMC8456889.
- Okuda K, Dang H, Kobayashi Y, Carraro G, Nakano S, Chen G, Kato T, Asakura T, Gilmore RC, Morton LC, Lee RE, Mascenik T, Yin WN, Barbosa Cardenas SM, O’Neal YK, Minnick CE, Chua M, Quinney NL, Gentzsch M, Anderson CW, Ghio A, Matsui H, Nagase T, Ostrowski LE, Grubb BR, Olsen JC, Randell SH, Stripp BR, Tata PR, O’Neal WK, Boucher RC. Secretory Cells Dominate Airway CFTR Expression and Function in Human Airway Superficial Epithelia. Am J Respir Crit Care Med. 2021 May 15;203(10):1275-1289. doi: 10.1164/rccm.202008-3198OC. PMID: 33321047. PMCID: PMC8456462.
- Carraro G, Langerman J, Sabri S, Lorenzana Z, Purkayastha A, Zhang G, Konda B, Aros CJ, Calvert BA, Szymaniak A, Wilson E, Mulligan M, Bhatt P, Lu J, Vijayaraj P, Yao C, Shia DW, Lund AJ, Israely E, Rickabaugh TM, Ernst J, Mense M, Randell SH, Vladar EK, Ryan AL, Plath K, Mahoney JE, Stripp BR, Gomperts BN. Transcriptional analysis of cystic fibrosis airways at single-cell resolution reveals altered epithelial cell states and composition. Nat Med. 2021 May;27(5):806-814. doi: 10.1038/s41591-021-01332-7. PMID: 33958799.
- He L, Kennedy AS, Houck S, Aleksandrov A, Quinney NL, Cyr-Scully A, Cholon DM, Gentzsch M, Randell SH, Ren HY, Cyr DM. DNAJB12 and Hsp70 Triage Arrested Intermediates of N1303K-CFTR for ER Associated-Autophagy. Mol Biol Cell. 2021 Apr 1;32(7):538-553. PMID: 33534640. PMCID: PMC8101465.
- Hawkins FJ, Suzuki S, Beermann ML, Barillà C, Wang R, Villacorta-Martin C, Berical A, Jean JC, Le Suer J, Matte T, Simone-Roach C, Tang Y, Schlaeger TM, Crane AM, Matthias N, Huang SXL, Randell SH, Wu J, Spence JR, Carraro G, Stripp BR, Rab A, Sorsher EJ, Horani A, Brody SL, Davis BR, Kotton DN. Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells. Cell Stem Cell. 2021 Jan 7;28(1):79-95.e8. doi: 10.1016/j.stem.2020.09.017. PMID: 33098807; PMCID: PMC7796997.
- Hou YJ, Chiba S, Halfmann P, Ehre C, Kuroda M, Dinnon KH 3rd, Leist SR, Schäfer A, Nakajima N, Takahashi K, Lee RE, Mascenik TM, Graham R, Edwards CE, Tse LV, Okuda K, Markmann AJ, Bartelt L, de Silva A, Margolis DM, Boucher RC, Randell SH, Suzuki T, Gralinski LE, Kawaoka Y, Baric RS. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020 Dec 18;370(6523):1464-1468. doi: 10.1126/science.abe8499. PMID: 33184236. PMCID: PMC7775736.
- Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, Konkimalla A, Asakura T, Mikami Y, Fritch EJ, Lee PJ, Heaton NS, Boucher RC, Randell SH, Baric RS, Tata PR. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell. 2020 Dec 3;27(6):890-904.e8. doi: 10.1016/j.stem.2020.10.005. PMID: 33128895; PMCID: PMC7577733.
- Carraro G, Mulay A, Yao C, Mizuno T, Konda B, Petrov M, Lafkas D, Arron JR, Hogaboam CM, Chen P, Jiang D, Noble PW, Randell SH, McQualter JL, Stripp BR. Single-Cell Reconstruction of Human Basal Cell Diversity in Normal and Idiopathic Pulmonary Fibrosis Lungs. Am J Respir Crit Care Med. 2020 Dec 1;202(11):1540-1550. doi: 10.1164/rccm.201904-0792OC. PMID: 32692579; PMCID: PMC7706153.
- Sigmon JS, Blanchard MW, Baric RS, Bell TA, Brennan J, Brockmann GA, Burks AW, Calabrese JM, Caron KM, Cheney RE, Ciavatta D, Conlon F, Darr DB, Faber J, Franklin C, Gershon TR, Gralinski L, Gu B, Gaines CH, Hagan RS, Heimsath EG, Heise MT, Hock P, Ideraabdullah F, Jennette JC, Kafri T, Kashfeen A, Kulis M, Kumar V, Linnertz C, Livraghi-Butrico A, Lloyd KCK, Lutz C, Lynch RM, Magnuson T, Matsushima GK, McMullan R, Miller DR, Mohlke KL, Moy SS, Murphy CEY, Najarian M, O’Brien L, Palmer AA, Philpot BD, Randell SH, Reinholdt L, Ren Y, Rockwood S, Rogala AR, Saraswatula A, Sassetti CM, Schisler JC, Schoenrock SA, Shaw GD, Shorter JR, Smith CM, St Pierre CL, Tarantino LM, Threadgill DW, Valdar W, Vilen BJ, Wardwell K, Whitmire JK, Williams L, Zylka MJ, Ferris MT, McMillan L, Manuel de Villena FP. Content and Performance of the MiniMUGA Genotyping Array: A New Tool To Improve Rigor and Reproducibility in Mouse Research. Genetics. 2020 Dec;216(4):905-930. doi: 10.1534/genetics.120.303596. PMID: 33067325; PMCID: PMC7768238.
- Harrison EB, Porrello A, Bowman BM, Belanger AR, Yacovone G, Azam SH, Windham IA, Ghosh SK, Wang M, McKenzie N, Waugh TA, Van Swearingen AED, Cohen SM, Allen DG, Goodwin TJ, Mascenik T, Bear JE, Cohen S, Randell SH, Massion PP, Major MB, Huang L, Pecot CV. A circle RNA regulatory axis promotes lung squamous metastasis via CDR1-mediated regulation of Golgi trafficking. Cancer Res. 2020 Nov 15;80(22):4972-4985. doi: 10.1158/0008-5472.CAN-20-1162. PMID: 32978168. PMCID: PMC7669576.
- Edwards CE, Yount BL, Graham RL, Leist SR, Hou YJ, Dinnon KH 3rd, Sims AC, Swanstrom J, Gully K, Scobey TD, Cooley MR, Currie CG, Randell SH, Baric RS. Swine acute diarrhea syndrome coronavirus replication in primary human cells reveals potential susceptibility to infection. Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):26915-26925. doi: 10.1073/pnas.2001046117. PMID: 33046644; PMCID: PMC7604506.
- Bowman BM, Montgomery SA, Schrank TP, Simon JM, Ptacek TS, Tamir TY, Mulvaney KM, Weir SJ, Nguyen TT, Murphy RM, Makowski L, Hayes DN, Chen XL, Randell SH, Weissman BE, Major MB. A conditional mouse expressing an activating mutation in NRF2 displays hyperplasia of the upper gastrointestinal tract and decreased white adipose tissue. J Pathol. 2020 Oct;252(2):125-137. doi: 10.1002/path.5504. PMID: 32619021; PMCID: PMC7511428.
- Kasam RK, Gajjala PR, Jegga AG, Courtney JA, Randell SH, Kramer EL, Clancy JP, Madala SK. Fibrocyte accumulation in the lungs of cystic fibrosis patients. J Cyst Fibros. 2020 Sep;19(5):815-822. doi: 10.1016/j.jcf.2020.06.011. PMID: 32593509; PMCID: PMC7492481.
- King NE, Suzuki S, Barillà C, Hawkins FJ, Randell SH, Reynolds SD, Stripp BR, Davis BR. Correction of Airway Stem Cells: Genome Editing Approaches for the Treatment of Cystic Fibrosis. Hum Gene Ther. 2020 Sep;31(17-18):956-972. doi: 10.1089/hum.2020.160. PMID: 32741223; PMCID: PMC7495916.
- Lee RE, Miller SM, Mascenik TM, Lewis CA, Dang H, Boggs ZH, Tarran R, Randell SH. Assessing Human Airway Epithelial Progenitor Cells for Cystic Fibrosis Cell Therapy. Am J Respir Cell Mol Biol. 2020 Sep;63(3):374-385. doi: 10.1165/rcmb.2019-0384OC. PMID: 32437238; PMCID: PMC7462339.
- McQueen BE, Kiatthanapaiboon A, Fulcher ML, Lam M, Patton K, Powell E, Kollipara A, Madden V, Suchland RJ, Wyrick P, O’Connell CM, Reidel B, Kesimer M, Randell SH, Darville T, Nagarajan UM. Human Fallopian Tube Epithelial Cell Culture Model To Study Host Responses to Chlamydia trachomatis Infection. Infect Immun. 2020 Aug 19;88(9):e00105-20. doi: 10.1128/IAI.00105-20. PMID: 32601108; PMCID: PMC7440757.
- Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon III KH, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Fulcher L, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O’Neal WK, Randell SH, Boucher RC, Baric RS. Reconstitution of SARS-CoV-2 infection from upper to lower respiratory tract. Cell. 2020 Jul 23;182(2):429-446.e14. doi: 10.1016/j.cell.2020.05.042. PMID: 32526206; PMCID: PMC7250779.
- Suzuki S, Crane AM, Anirudhan V, Barillà C, Matthias N, Randell SH, Rab A, Sorscher EJ, Kerschner JL, Yin S, Harris A, Mendel M, Kim K, Zhang L, Conway A, Davis BR. Highly Efficient Gene Editing of Cystic Fibrosis Patient-Derived Airway Basal Cells Results in Functional CFTR Correction. Mol Ther. 2020 Jul 8;28(7):1684-1695. doi: 10.1016/j.ymthe.2020.04.021. PMID: 32402246. PMCID: PMC7335734.
- Menachery VD, Dinnon KH 3rd, Yount BL Jr, McAnarney ET, Gralinski LE, Hale A, Graham RL, Scobey T, Anthony SJ, Wang L, Graham B, Randell SH, Lipkin WI, Baric RS. Trypsin treatment unlocks barrier for zoonotic bat coronaviruses infection. J Virol. 2020 Feb 14;94(5). doi: 10.1128/JVI.01774-19. PMID: 31801868. PMCID: PMC7022341.
- Kılıç A, Ameli A, Park JA, Kho AT, Tantisira K, Santolini M, Cheng F, Mitchel JA, McGill M, O’Sullivan MJ, De Marzio M, Sharma A, Randell SH, Drazen JM, Fredberg JJ, Weiss ST. Mechanical forces induce an asthma gene signature in healthy airway epithelial cells. Sci Rep. 2020 Jan 22;10(1):966. doi: 10.1038/s41598-020-57755-8. PMID: 31969610. PMCID: PMC6976696.
- Nguyen TT, Soma PS, Mascenik T, Lewis CA, Lee RE, Thorp BD, Zanation AM, Ebert CS Jr, Senior BA, Randell SH, Ehrmann BM, Kimple AJ. Mometasone absorption in cultured airway epithelium. Int Forum Allergy Rhinol. 2019 Dec;9(12):1451-1455. doi: 10.1002/alr.22441. PMID: 31633879. PMCID: PMC6927533.
- Smeekens JM, Immormino RM, Balogh PA, Randell SH, Kulis MD, Moran TP. Indoor dust acts as an adjuvant to promote sensitization to peanut through the airway. Clin Exp Allergy. 2019 Nov;49(11):1500-1511. doi: 10.1111/cea.13486. PMID: 31444814. PMCID: PMC7171466.
- Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson BD, Volmer AS, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CMP, O’Neal WK, Boucher RC. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest. 2019 Oct 1;129(10):4433-4450. doi: 10.1172/JCI125669. PMID: 31524632. PMCID: PMC6763234.
- Marozkina N, Bosch J, Cotton C, Smith L, Seckler J, Zaman K, Rehman S, Periasamy A, Gaston H, Altawallbeh G, Davis M, Jones DR, Schilz R, Randell SH, Gaston B. Cyclic compression increases F508 Del CFTR expression in ciliated human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2019 Aug 1;317(2):L247-L258. doi: 10.1152/ajplung.00020.2019. PMID: 31116581. PMCID: PMC6734384.
- Parker MM, Hao Y, Guo F, Pham B, Chase R, Platig J, Cho MH, Hersh CP, Thannickal VJ, Crapo J, Washko G, Randell SH, Silverman EK, San José Estépar R, Zhou X, Castaldi PJ. Identification of an emphysema-associated genetic variant near TGFB2 with regulatory effects in lung fibroblasts. Elife. 2019 Jul 25;8. doi: 10.7554/eLife.42720. PMID: 31343404. PMCID: PMC6693893.
- Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, Martino MB, Dang H, Abzhanova A, Lin JM, Hull-Ryde EA, Volmer AS, Randell SH, Livraghi-Butrico A, Deng Y, Scherer PE, Stripp BR, O’Neal WK, Boucher RC. XBP1S Regulates MUC5B in a Promoter Variant-Dependent Pathway in IPF Airway Epithelia. Am J Respir Crit Care Med. 2019 Jul 15;200(2):220-234. doi: 10.1164/rccm.201810-1972OC. PMID: 30973754. PMCID: PMC6635783.
- Barrios J, Kho AT, Aven L, Mitchel JA, Park JA, Randell SH, Miller LA, Tantisira KG, Ai X. Pulmonary Neuroendocrine Cells Secrete GABA to Induce Goblet Cell Hyperplasia in Primate Models. Am J Respir Cell Mol Biol. 2019 Jun;60(6):687-694. doi: 10.1165/rcmb.2018-0179OC. PMID: 30571139. PMCID: PMC6543741.
- Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, Radicioni G, Kesimer M, Chua M, Dang H, Livraghi-Butrico A, Ehre C, Doerschuk CM, Randell SH, Matsui H, Nagase T, O’Neal WK, Boucher RC. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am J Respir Crit Care Med. 2019 Mar 15;199(6):715-727. doi: 10.1164/rccm.201804-0734OC. PMID: 30352166. PMCID: PMC6423099.
- Berical A, Lee RE, Randell SH, Hawkins F. Challenges Facing Airway Epithelial Cell-Based Therapy for Cystic Fibrosis. Front Pharmacol. 2019 Feb 8;10:74. doi: 10.3389/fphar.2019.00074. PMID: 30800069. PMCID: PMC6376457.
- Khatri A, Kraft BD, Tata PR, Randell SH, Piantadosi CA, Pendergast AM. ABL kinase inhibition promotes lung regeneration through expansion of an SCGB1A1+ SPC+ cell population following bacterial pneumonia. Proc Natl Acad Sci U S A. 2019 Jan 29;116(5):1603-1612. doi: 10.1073/pnas.1816030116. PMID: 30655340. PMCID: PMC6358689.
- Panganiban RA, Sun M, Dahlin A, Park HR, Kan M, Himes BE, Mitchel JA, Iribarren C, Jorgenson E, Randell SH, Israel E, Tantisira K, Shore S, Park JA, Weiss ST, Wu AC, Lu Q. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J Allergy Clin Immunol. 2018 Nov;142(5):1469-1478.e2. doi: 10.1016/j.jaci.2017.11.040. PMID: 29330013. PMCID: PMC6037620.
- Gupta R, Radicioni G, Abdelwahab S, Dang H, Carpenter J, Chua M, Mieczkowski PA, Sheridan J, Randell SH, Kesimer M. Intercellular Communication between Airway Epithelial Cells is Mediated by Exosome-Like Vesicles. Am J Respir Cell Mol Biol. 2019 Feb;60(2):209-220. doi: 10.1165/rcmb.2018-0156OC. PMID: 30230353. PMCID: PMC6376407.
- Ghosh A, Coakley RC, Mascenik T, Rowell TR, Davis ES, Rogers K, Webster MJ, Dang H, Herring LE, Sassano MF, Livraghi-Butrico A, Van Buren SK, Graves LM, Herman MA, Randell SH, Alexis NE, Tarran R. Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018 Jul 1;198(1):67-76. doi: 10.1164/rccm.201710-2033OC. PMID: 29481290. PMCID: PMC6034122.
- Gillen AE, Yang R, Cotton CU, Perez A, Randell SH, Leir SH, Harris A. Molecular characterization of gene regulatory networks in primary human tracheal and bronchial epithelial cells. J Cyst Fibros. 2018 Jul;17(4):444-453. doi: 10.1016/j.jcf.2018.01.009. PMID: 29459038. PMCID: PMC6026051.
- Porrello A, Leslie PL, Harrison EB, Gorentla BK, Kattula S, Ghosh SK, Azam SH, Holtzhausen A, Chao YL, Hayward MC, Waugh TA, Bae S, Godfrey V, Randell SH, Oderup C, Makowski L, Weiss J, Wilkerson MD, Hayes DN, Earp HS, Baldwin AS, Wolberg AS, Pecot CV. Factor XIIIA-expressing inflammatory monocytes promote lung squamous cancer through fibrin cross-linking. Nat Commun. 2018 May 18;9(1):1988. doi: 10.1038/s41467-018-04355-w. PMID: 29777108. PMCID: PMC5959879.
- Terryah ST, Fellner RC, Ahmad S, Moore PJ, Reidel B, Sesma JI, Kim CS, Garland AL, Scott DW, Sabater JR, Carpenter J, Randell SH, Kesimer M, Abraham WM, Arendshorst WJ, Tarran R. Evaluation of a SPLUNC1-derived peptide for the treatment of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2018 Jan 1;314(1):L192-L205. doi: 10.1152/ajplung.00546.2016. PMID: 28982737. PMCID: PMC5866433.
- Yu D, Saini Y, Chen G, Ghio AJ, Dang H, Burns KA, Wang Y, Davis RM, Randell SH, Esther Jr CR, Paulsen F, Boucher RC. Loss of β epithelial sodium channel function in meibomian glands produces pseudohypoaldosteronism 1-like ocular disease in mice. Am J Pathol. 2018 Jan;188(1):95-110.doi: 10.1016/j.ajpath.2017.09.016. PMID: 29107074. PMCID: PMC5745530.
- Keating JE, Minges JT, Randell SH, Glish GL. Paper spray mass spectrometry for high-throughput quantification of nicotine and cotinine. Anal Methods. 2018;10(1):46-50. doi: 10.1039/C7AY02204B. PMID: 29568335. PMCID: PMC5858713.
- Randell SH, Zeldin DC. A slippery cause of a slimy problem: Mucin induction by an esterified lipid. Am J Respir Cell Mol Biol. 2017 Dec;57(6):633-634. doi: 10.1165/rcmb.2017-0275ED. PMID: 29192828. PMCID: PMC5800897.
- Snyder RJ, Hussain S, Tucker CJ, Randell SH, Garantziotis S. Impaired Ciliogenesis in differentiating human bronchial epithelia exposed to non-Cytotoxic doses of multi-walled carbon Nanotubes. Part Fibre Toxicol. 2017 Nov 13;14(1):44. doi: 10.1186/s12989-017-0225-1. PMID: 29132433. PMCID: PMC5683528.
- Venkataraman A, Blackwell JW, Funkhouser WK, Birchard KR, Beamer SE, Simmons WT, Randell SH, Egan TM. Beware cold agglutinins in organ donors! Ex vivo lung perfusion from an uncontrolled donation after circulatory-determination-of-death donor with a cold agglutinin: A case report. Transplant Proc. 2017 Sep;49(7):1678-1681. doi: 10.1016/j.transproceed.2017.04.004. PMID: 28838463. PMCID: PMC6034983.
- Fossum SL, Mutolo MJ, Tugores A, Ghosh S, Randell SH, Jones LC, Leir SH, Harris A. Ets homologous factor (EHF) has critical roles in epithelial dysfunction in airway disease. J Biol Chem. 2017 Jun 30;292(26):10938-10949. doi: 10.1074/jbc.M117.775304. PMID: 28461336. PMCID: PMC5491778.
- Xiang H, Yuan L, Gao X, Alexander PB, Lopez O, Lau C, Ding Y, Chong M, Sun T, Chen R, Liu SQ, Wu H, Wan Y, Randell SH, Li QJ, Wang XF. UHRF1 is required for basal stem cell proliferation in response to airway injury. Cell Discov. 2017 Jun 13;3:17019. doi: 10.1038/celldisc.2017.19. PMID: 28626588. PMCID: PMC5468773.
- Gentzsch M, Boyles SE, Cheluvaraju C, Chaudhry IG, Quinney NL, Cho C, Dang H, Liu X, Schlegel R, Randell SH. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2017 May;56(5):568-574. doi: 10.1165/rcmb.2016-0276MA. PMID: 27983869. PMCID: PMC5449492.
- Anthony SJ, Gilardi K, Menachery VD, Goldstein T, Ssebide B, Mbabazi R, Navarrete-Macias I, Liang E, Wells H, Hicks A, Petrosov A, Byarugaba DK, Debbink K, Dinnon KH, Scobey T, Randell SH, Yount BL, Cranfield M, Johnson CK, Baric RS, Lipkin WI, Mazet JA. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. MBio. 2017 Apr 4;8(2). doi: 10.1128/mBio.00373-17. PMID: 28377531. PMCID: PMC5380844.
- Liu X, Krawczyk E, Suprynowicz FA, Palechor-Ceron N, Yuan H, Dakic A, Simic V, Zheng YL, Sripadhan P, Chen C, Lu J, Hou TW, Choudhury S, Kallakury B, Tang DG, Darling T, Thangapazham R, Timofeeva O, Dritschilo A, Randell SH, Albanese C, Agarwal S, Schlegel R. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat Protoc. 2017 Feb;12(2):439-451. doi: 10.1038/nprot.2016.174. PMID: 28125105. PMCID: PMC6195120.
- Mitchel JA, Antoniak S, Lee JH, Kim SH, McGill M, Kasahara DI, Randell SH, Israel E, Shore SA, Mackman N, Park JA. IL-13 augments compressive stress-induced tissue factor expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2016 Apr;54(4):524-31. doi: 10.1165/rcmb.2015-0252OC. PMID: 26407210. PMCID: PMC4821058.
- Gentzsch M, Ren HY, Houck SA, Quinney NL, Cholon DM, Sopha P, Chaudhry IG, Das J, Dokholyan NV, Randell SH, Cyr DM. Restoration of R117H CFTR folding and function in human airway cells through combination treatment with VX-809 and VX-770. Am J Physiol Lung Cell Mol Physiol. 2016 Sep 1;311(3):L550-9. doi: 10.1152/ajplung.00186. PMID: 27402691. PMCID: PMC5142211.
- Wagner DE, Cardoso WV, Gilpin SE, Majka S, Ott H, Randell SH, Thébaud B, Waddell T, Weiss DJ; ATS Subcommittee on Stem Cells and Cell Therapies. An Official American Thoracic Society Workshop Report 2015. Stem cells and cell therapies in lung biology and diseases. Ann Am Thorac Soc. 2016 Aug;13(8):S259-78. doi: 10.1513/AnnalsATS.201606-466ST. PMID: 27509163.
- Yu D, Davis RM, Aita M, Burns KA, Clapp PW, Gilmore RC, Chua M, O’Neal WK, Schlegel R, Randell SH, C Boucher R. Characterization of rat meibomian gland ion and fluid transport. Invest Ophthalmol Vis Sci. 2016 Apr 1;57(4):2328-43. doi: 10.1167/iovs.15-17945. PMID: 27127933. PMCID: PMC4855829.
- Menachery VD, Yount BL Jr, Sims AC, Debbink K, Agnihothram SS, Gralinski LE, Graham RL, Scobey T, Plante JA, Royal SR, Swanstrom J, Sheahan TP, Pickles RJ, Corti D, Randell SH, Lanzavecchia A, Marasco WA, Baric RS. SARS-like WIV1-CoV poised for human emergence. Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):3048-53. doi: 10.1073/pnas.1517719113. PMID: 26976607. PMCID: PMC4801244.
- Park JA, Mitchel JA, Qazvini NT, Kim JH, Park CY, Butler JP, Israel E, Randell SH, Shore SA, Drazen JM, Fredberg JJ. Compressive stress causes an unjamming transition and an epithelial-mesenchymal transition in the airway epithelium in asthma. Ann Am Thorac Soc. 2016 Mar;13 Suppl 1:S102. doi: 10.1513/AnnalsATS.201506-382MG. PMID: 27027941. PMCID: PMC5015726.
- Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, Ostrowski LE, Abbott-Banner K, Hanrahan JW. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2016 Jan 1;310(1):L59-70. doi: 10.1152/ajplung.00324.2015. PMID: 26545902.
- Park JA, Kim JH, Bi D, Mitchel JA, Qazvini NT, Tantisira K, Park CY, McGill M, Kim SH, Gweon B, Notbohm J, Steward R Jr, Burger S, Randell SH, Kho AT, Tambe DT, Hardin C, Shore SA, Israel E, Weitz DA, Tschumperlin DJ, Henske EP, Weiss ST, Manning ML, Butler JP, Drazen JM, Fredberg JJ. Unjamming and cell shape in the asthmatic airway epithelium. Nat Mater. 2015 Oct;14(10):1040-8. doi: 10.1038/nmat4357. PMID: 26237129. PMCID: PMC4666305.
- Evans ES, Hackney AC, McMurray RG, Randell SH, Muss HB, Deal AM, Battaglini CL. Impact of acute intermittent exercise on natural killer cells in breast cancer survivors. Integr Cancer Ther. 2015 Sep;14(5):436-45. doi: 10.1177/1534735415580681. PMID: 25873292.
- Fritzsching B, Zhou-Suckow Z, Trojanek JB, Schubert SC, Schatterny J, Hirtz S, Agrawal R, Muley T, Kahn N, Sticht C, Gunkel N, Welte T, Randell SH, Länger F, Schnabel P, Herth FJ, Mall MA. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015 Apr 15;191(8):902-13. doi: 10.1164/rccm.201409-1610OC. PubMed PMID: 25607238; PubMed Central PMCID: PMC4435455.
- Chand HS, Montano G, Huang X, Randell SH, Mebratu Y, Petersen H, Tesfaigzi Y. A genetic variant of p53 restricts the mucous secretory phenotype by regulating SPDEF and Bcl-2 expression. Nat Commun. 2014 Nov 27;5:5567. doi: 10.1038/ncomms6567. PubMed PMID: 25429397; PubMed Central PMCID: PMC4247165.
- Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014 Aug 7;15(2):123-38. doi: 10.1016/j.stem.2014.07.012. Review. PubMed PMID: 25105578; PubMed Central PMCID: PMC4212493.
- Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med. 2014 Jul 23;6(246):246ra96. doi: 10.1126/scitranslmed.3008680. PubMed PMID: 25101886; PubMed Central PMCID: PMC4272825.
- Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014 Feb 1;189(3):301-13. doi: 10.1164/rccm.201306-1181OC. PubMed PMID: 24392884; PubMed Central PMCID: PMC3977731.
