Measures of the Pulmonary Microbiome
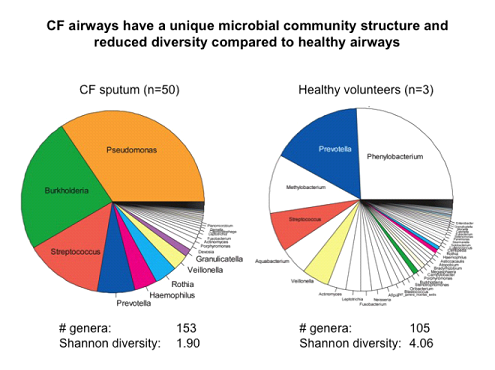
UNC has developed the capacity to measure the respiratory microbiome with 16S-454 pyrosequencing technologies (Fig. 22). This technique has already been applied to studies of acute exacerbations in CF and COPD and measurement of antibiotic effects.
In sum, the Marsico Lung Institute has a diverse array of capabilities for performing first-in-man Phase I clinical studies of novel therapeutic agents and is highly motivated to perform such studies.
 |
|---|
|
Figure 22. 16S pyrosequencing analyses of human respiratory microbiome. Distribution of microbiome of CF (L) and normal (R) induced sputum as determined by 16S-454 pyrosequencing. |