Collaboration
The Marsico Lung Institute has an extensive history of taking small molecules and biologics from the “bench” through the IND process and Phase I testing in man. It has initiated more than 10 investigator-initiated INDs for first-in-man testing of potential therapeutic agents for CF and COPD. Hence, there has been a broad range of preclinical efficacy, pharmacokinetic, and safety models important for target validation and useful for IND submissions. These capabilities are complemented by an extensive array of Phase I clinical testing capabilities, including novel biomarker, pharmacokinetic, and imaging techniques, coupled with the appropriate IND/regulatory infrastructure. In parallel, the Marsico Lung Institute has a large experience with Phase II/III clinical studies with industry.
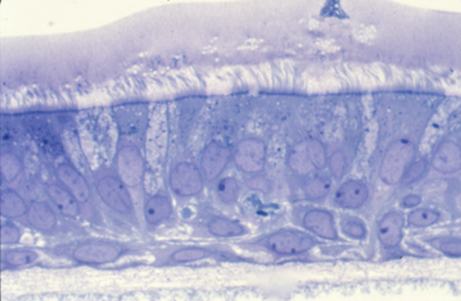
Figure 1. Human lung primary bronchial epithelial cells in air liquid interface cultures. The cells were prepared for histology using perflurocarbon-OsO4 to preserve the apical periciliary layer and overlying mucus.
Collaboration Capabilities:
If you are interested in learning more about how the UNC CF Center can help further your research, or are interested in collaborating with us, please contact our Director, Dr. Richard Boucher:
7008 Marsico Hall
The University of North Carolina at Chapel Hill
Campus Box #7248
Phone: (919) 966-1077
Fax: (919) 966-5178
Email: richard_boucher@med.unc.edu