Novel Biomarkers for Pulmonary Drug Development
The Marsico Lung Institute has developed a number of novel biomarkers for measuring the pharmacodynamic and therapeutic effects of inhaled therapeutic agents in sputum. Much effort has been focused on the measurement of the proper vs. improper hydration of mucus in airway diseases. Consequently, the Marsico Lung Institute has a spectrum of technologies designed to measure these parameters in induced or expectorated sputum, including measures of absolute mucin concentrations, mucin/mucus osmotic pressure/modulus, and measures of mucus adhesion (Fig. 21). In parallel, the Marsico Lung Institute has the capacity to measure a large number of classical biomarkers in sputum through our Sputum Core, including measures of cell counts, cytokines, and growth factors.
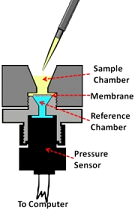 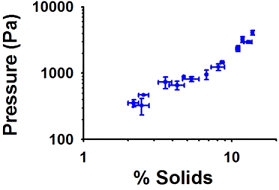 |
| Figure 21. A. Custom-designed oncometer system used to measure the osmotic pressure/moduli of human airway mucus. B. Summary data showing the osmotic pressure vs. mucus concentration (% solids). |