Novel Airway Surface Pharmacokinetics Techniques
Measurement of the pharmacokinetics of drugs on the airway surface is an important but difficult technique to make practical for drug development. The Marsico Lung Institute has an extensive experience in bronchoscopy of subjects post-drug delivery and direct (filter paper) and directed lavage techniques for making such measurements. More recently, much effort has been dedicated to developing a non-invasive technique that is suitable for repetitive PK measurements to generate accurate measurements of pharmacokinetic parameters. A technique that has generated much promise in this regard is the exhaled breath condensate (EBC) technique that harvests small quantities of aerosol droplets, presumably generated in small airways at inspiration, that can be analyzed with high sensitivity by mass spectroscopy approaches. Indeed, the ability to measure the drug, coupled with the capacity to get an absolute concentration of the drug in airway surface liquid by concomitant mass spectroscopy measurement of urea concentrations, offers a powerful and novel technique to make these aforesaid measurements (Fig. 20).
|
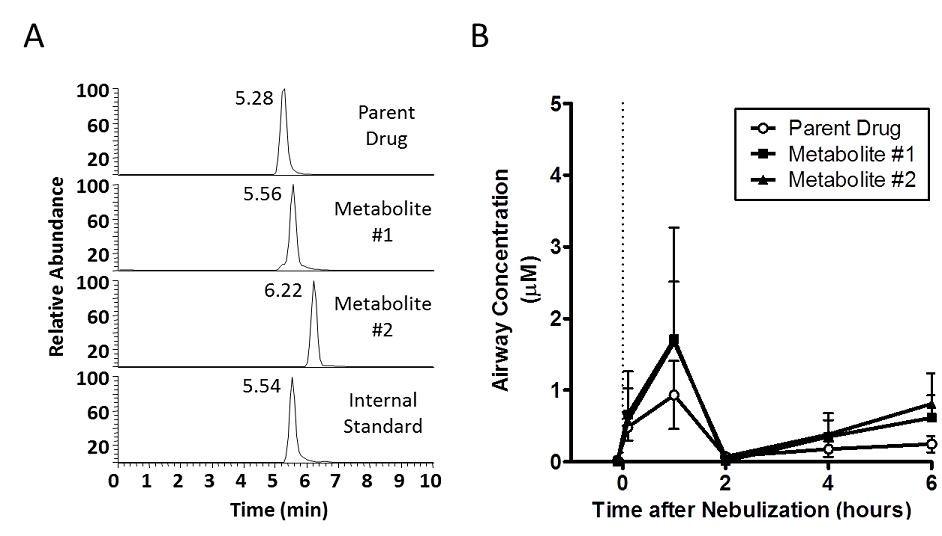
Figure 20. Monitoring of airway drug concentrations. A. Mass spectrometry was utilized to detect parent drug and two primary metabolites of a novel inhaled sodium channel antagonist, plus a related compound as an internal standard. The dilution marker urea and other native metabolites were detected simultaneously as previously described (Esther et al., Rapid Comm Mass Spec, 2009.) B. Airway concentrations of parent drug and metabolites after nebulization in a animal model were assessed through analysis of exhaled breath condensate (EBC). Drug, metabolite, and urea concentrations in EBC collected at various intervals were measured using mass spectrometry, and dilution of airway secretions in EBC was calculated using EBC:serum urea ratios.
|
Figure 20. Monitoring of airway drug concentrations. A. Mass spectrometry was utilized to detect parent drug and two primary metabolites of a novel inhaled sodium channel antagonist, plus a related compound as an internal standard. The dilution marker urea and other native metabolites were detected simultaneously as previously described (Esther et al., Rapid Comm Mass Spec, 2009). B. Airway concentrations of parent drug and metabolites after nebulization in an animal model were assessed through analysis of exhaled breath condensate (EBC). Drug, metabolite, and urea concentrations in EBC collected at various intervals were measured using mass spectrometry, and dilution of airway secretions in EBC was calculated using EBC:serum urea ratios.