Ion/Liquid Transport
The Marsico Lung Institute also has extensive capabilities to perform classical Ussing chamber studies on large numbers of tissues for screening purposes. Extensive experience has accrued in developing the appropriate quality control procedures to assure accurate and reproducible testing of compound activities in this assay.
The Institute also has extensive experience in characterizing airway surface liquid physiology, in response to candidate therapeutic agents, under the “thin film” ASL technique (Fig. 6). In “standard” versions of this technique, a combination of airway surface liquid (ASL) volume as measured by confocal techniques, indices of ion transport processes mediating ASL volume homeostasis from transepithelial microelectrode voltage measurements, coupled with micropipette liquid sampling for measurements of drug concentrations, can be made. Note, drugs can be added to the basolateral compartment of these preparations, the luminal compartment in perfluorocarbon, or indeed, more recently, by an interface to ultrasonic nebulizers that deliver aerosols to cultured airway surfaces at rates that mimic aerosol deposition in vivo (e.g., 20 nl/cm2/min – Fig. 7).
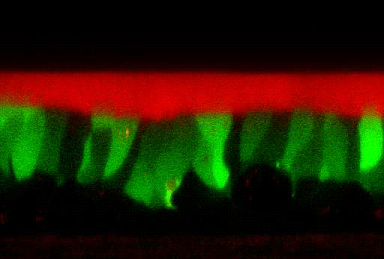
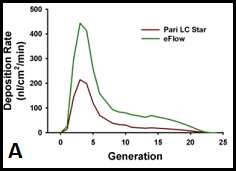
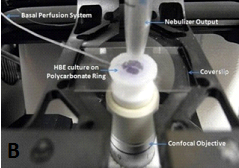
Figure 7. Interface of HBE cultures with aerosol delivery of agents. A. The average deposition rate (nanoliters per square cm per minute of deposition) over the first 20 airway generations in humans, comparing the rates of two commercially available nebulizers. B. Nano-liter volume nebulization system positioned for drug deposition to the apical surface of human bronchial epithelial cells.
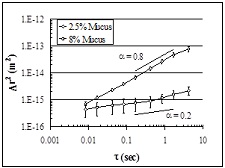 |
| Figure 8. Diffusion of microbeads through HBE mucus. Movement [i.e., mean squared displacement, Δr2(m2)] ~1μ particle is faster in normal 1% mucus than CF-like, dehydrated/thickened mucus. |