
1 week ago

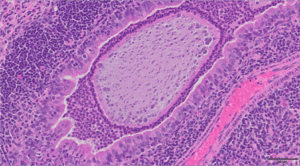
The image above is a representative H&E histological section of a bacteria-laden alginate bead in the airway of a mouse infected with Pseudomonas aeruginosa detected 28 days post infection. Scale bar = 50μm.
This image is from the Wolfgang Lab and was taken by Matthew Greenwald, PhD in collaboration with Alessandra Livraghi-Butrico.

1 week ago

1 week ago

1 week ago

2 weeks ago