In a groundbreaking study from our department, researchers explored into the intricate world of mechanical responses within mammalian cells and tissues. The team, led by Dr. Sharon L Campbell, in collaboration with Dr. Brenton D. Hoffman lab at the Duke University focused on understanding how load-bearing proteins can dynamically resist or accommodate mechanical forces in different directions. Specifically, their work centered on elucidating the phenomenon of directional catch bonds between F-actin and vinculin (Vcn) in cell-ECM adhesion. Employing an innovative approach that integrated Molecular Dynamics Simulations, rational engineering, biochemical validation, and FRET-based biosensor studies, the researchers successfully identified and characterized amino acid variants mediating directional catch bonding. The results, published in Nature Communications, shed light on the nuanced role of Vcn in influencing cell substructures and overall cellular behavior, with minimal impact on other cellular functions. This interdisciplinary investigation not only advances our understanding of the molecular mechanisms behind directional catch bonding but also holds promise for applications in cell–ECM adhesion, single-cell migration, and potentially in broader cellular processes such as cell–cell adhesion and collective migration in both developmental and pathophysiological contexts.
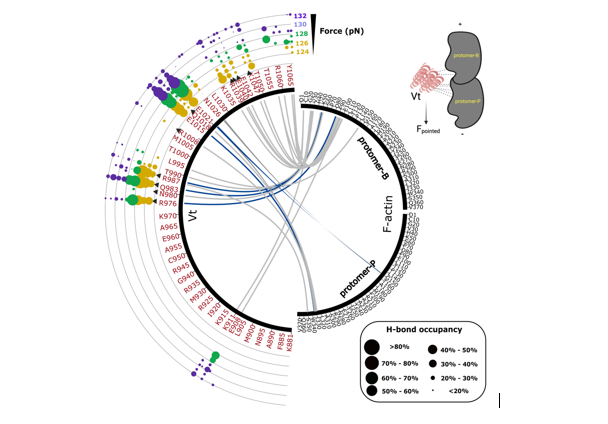
Evaluation of energetics and hydrogen bond properties between F-actin and Vt
Link to publication:
Chirasani, V.R., Khan, M.A.I., Malavade, J.N. et al. Molecular basis and cellular functions of vinculin-actin directional catch bonding. Nat Commun 14, 8300 (2023).
Tags: Campbell lab and the R.L. Juliano Structural Bioinformatics Core
