
Congratulations to Rodney Park Ph.D. (2022 alum) who has a first-author publication “Designer installation of a substrate recruitment domain to tailor enzyme specificity” which was published in Nature Chemical Biology on December 12, 2022.
The team used protein design to create a novel multidomain enzyme that uses substrate scaffolding to enhance enzyme efficiency and selectivity.
Abstract
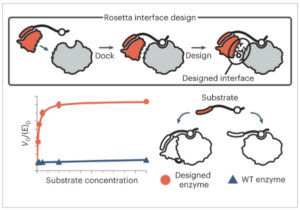 Promiscuous enzymes that modify peptides and proteins are powerful tools for labeling biomolecules; however, directing these modifications to desired substrates can be challenging. Here, we use computational interface design to install a substrate recognition domain adjacent to the active site of a promiscuous enzyme, catechol O-methyltransferase. This design approach effectively decouples substrate recognition from the site of catalysis and promotes modification of peptides recognized by the recruitment domain. We determined the crystal structure of this novel multidomain enzyme, SH3-588, which shows that it closely matches our design. SH3-588 methylates directed peptides with catalytic efficiencies exceeding the wild-type enzyme by over 1,000-fold, whereas peptides lacking the directing recognition sequence do not display enhanced efficiencies. In competition experiments, the designer enzyme preferentially modifies directed substrates over undirected substrates, suggesting that we can use designed recruitment domains to direct post-translational modifications to specific sequence motifs on target proteins in complex multisubstrate environments.
Promiscuous enzymes that modify peptides and proteins are powerful tools for labeling biomolecules; however, directing these modifications to desired substrates can be challenging. Here, we use computational interface design to install a substrate recognition domain adjacent to the active site of a promiscuous enzyme, catechol O-methyltransferase. This design approach effectively decouples substrate recognition from the site of catalysis and promotes modification of peptides recognized by the recruitment domain. We determined the crystal structure of this novel multidomain enzyme, SH3-588, which shows that it closely matches our design. SH3-588 methylates directed peptides with catalytic efficiencies exceeding the wild-type enzyme by over 1,000-fold, whereas peptides lacking the directing recognition sequence do not display enhanced efficiencies. In competition experiments, the designer enzyme preferentially modifies directed substrates over undirected substrates, suggesting that we can use designed recruitment domains to direct post-translational modifications to specific sequence motifs on target proteins in complex multisubstrate environments.
Link to publication
Park, R., Ongpipattanakul, C., Nair, S.K. et al. Designer installation of a substrate recruitment domain to tailor enzyme specificity. Nat Chem Biol (2022). https://doi.org/10.1038/s41589-022-01206-0
Connect with Dr. Rodney Park on Linked in
