In the journal Nature, Greg Wang, PhD, assistant professor of Biochemistry and Biophysics and Cell biology Program, and colleagues reported findings about the structure and function of the DNMT3A enzyme complex, which helps control gene expression.

Scientists at the University of North Carolina and University of California, Riverside, have unveiled new findings about the structure and function of an enzyme that is commonly mutated in blood disorders and cancers, including acute myeloid leukemia.
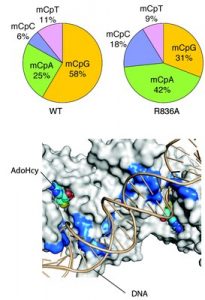
In the journal Nature, the researchers reported findings about the structure and function of the DNMT3A enzyme complex, which helps control gene expression by organizing DNA. The researchers at the University of California, Riverside, described the structure of DNMT3A, and researchers at UNC Lineberger took the lead on research into how mutations impact the enzyme’s function in the cell. They identified how this enzyme places a type of “chemical tag” at specific DNA sequences, and how mutations in the enzyme can lead to a change in the development course for the cell, as seen in cancer and developmental disorders.
“Now, because we understand the structure of this enzyme and its interaction with DNA, we see where exactly the cancer-related mutations often occur,” said Greg Wang, PhD, an assistant professor in the UNC School of Medicine Department of Biochemistry & Biophysics and Cell biology Program. “Following this lead, we conducted functional studies to show that when the enzyme has a mutation that affects the enzyme’s ability to bind with the DNA, it changes patterns of chemical ‘tags’ in DNA, affecting the future fate of the cell’s development, and sometimes, leading to cancer.”
UNC researchers studied a class of so-called DNA-binding-defective mutations of DNMT3A detected in human blood cancers, and found that when they put a mutant enzyme into a leukemia cell line, it caused a cancerous phenotype.
The findings improve upon our current understanding of this enzyme in disease development. Previously, the same group of UNC researchers showed in a study published in the journal Cancer Cell in 2016 that a specific mutation in the DNMT3A gene interferes with the protein-to-protein interaction interface of the enzyme complex, contributing to progression of blood cancer.
“In this current study, we show cancer cells also often gain somatic mutations to change the interaction of this enzyme, DNMT3A, with DNA in order to perturb DNA methylation and gene expression programs,” Wang said.
Normally, DNMT3A helps to maintain the patterns of “chemical tags” in the DNA to keep certain genes in check. UNC Lineberger researchers have now unveiled the two distinct mechanisms (Cancer Cell 2016 July; Nature 2018 Feb) by which somatic mutations of DNMT3A cause gene regulation defects leading to development of disease, notably blood cancer.
The research was supported by Kimmel Scholar Awards, the March of Dimes Foundation, the U.S. Department of Defense Cancer Research Program, Gabrielle’s Angel Foundation to Cancer Research, Gilead Sciences Research Scholars Program, the University Cancer Research Fund, and the National Institutes of Health.
The other authors were Zhi-Min Zhang, Rui Lu, Pengcheng Wang, Yang Yu, Dongliang Chen, Linfeng Gao, Shuo Liu, Debin Ji, Scott B. Rothbart, Yinsheng Wang, and Jikui Song.
Story courtesy of Laura Oleniacz, Science Communications Manager UNC Lineberger
BCBP Media contact: Carolyn M. Clabo, 919-962-7642, carolyn_clabo@med.unc.edu