

Soma Sengupta, MD, PhD, MBA, FRCP, FAAN, FANA
Soma Sengupta, MD, PhD, MBA, FRCP, FAAN, FANA
Division Chief, Neuro-oncology
Vice Chair, Neurology.
Professor
The University of North Carolina has a robust brain tumor research program. Well over a dozen labs in the Lineberger Comprehensive Cancer Center explore the diagnosis and novel treatment of brain tumors. Immunotherapy and cellular therapy are actively being investigated both in the laboratory setting and clinical trials. There is particular institutional expertise in genetic profiling of brain tumors, the study of IDH1, IDH1’s role in brain tumors and advanced imaging of brain tumors within the Biomedical Research Imaging Center (BRIC).
the diagnosis and novel treatment of brain tumors. Immunotherapy and cellular therapy are actively being investigated both in the laboratory setting and clinical trials. There is particular institutional expertise in genetic profiling of brain tumors, the study of IDH1, IDH1’s role in brain tumors and advanced imaging of brain tumors within the Biomedical Research Imaging Center (BRIC).
Glioblastoma (GBM) is the most common and aggressive primary brain tumor in adults. The standard of care for glioblastoma remains unchanged since 2005. Dr. Soma Sengupta and Dr. Yasmeen Rauf research focus is on the management and treatment of GBM. While Dr Sengupta focuses on commercial clinical trials and Dr. Rauf on cellular therapy clinical trials, both neuro oncologists collaborate in their research, and in the treatment of brain tumor patients at UNC Health.
Dr. Colette Shen currently leads the TRIDENT study for GBM patients at UNC Health, evaluating the safety and efficacy of Tumor Treating Fields (TTFields) concurrent with standard of care therapies.
TRIDENT Clinical Trial Contact:
Camisha Johnson
Phone: 919-445-4847
camisha_johnson@med.unc.edu
Dr. Yasmeen Rauf is leading clinical trials to determine a more effective way to treat patients with GBM. The study will evaluate the safety and tolerability of CAR-T immunotherapy, a very promising therapy for patients with recurrent or progressive glioblastoma.
CAR-T Clinical Trial Contact:
Catherine Cheng
Phone: 919-445-4208
catherine_cheng@med.unc.edu
UNC is enrolling patients in the ROADS clinical study. This clinical study compares 2 FDA-cleared radiation treatments that are proven safe and effective: GammaTile® Surgically Targeted Radiation Therapy (STaRT) and stereotactic radiotherapy (SRT).
ROADS Clinical Study Contact:
Dominique Higgins
Phone: 984-974-4175
higginsd@unc.edu
Imvax Phase 2b Clinical Trial of IGV-001 Contact:
Camisha Johnson
Phone: 919-445-4847
camisha_johnson@med.unc.edu
What is CAR-T immunotherapy?
CAR-T is a process that involves extracting T cells from patients, manufacturing the cells in the lab to identify tumor cells displaying a specific molecular target, and then re-infusing the cells to fight a patient’s cancer.
In human beings, when the immune system finds an abnormal cell, it creates a millions of T cells to kill the abnormal cell, trying not to harm the healthy cells. T cells have receptors on its surface. These claw-like receptors attach to the antigen. Once they attach, if they identify the antigen presenting cell as abnormal, it releases toxins to damage the cell. Cancer cells evade this immune response. CAR- T immunotherapy is used to help our body’s natural defense army to recognize cancer cells as abnormal cells and mound a response against them.
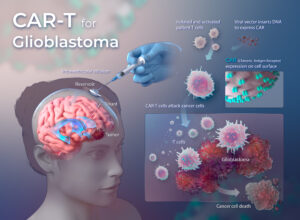
Modified and inactive viruses are used to change genetic information in a patient’s own T cell. The T cells are then programmed so that they now have ‘Chimeric Antigen Receptors’. The new receptors enable the T cells to latch onto the patient-specific antigen on the tumor cells and destroy them.
CAR-T immunotherapy at UNC Lineberger is one of a select few academic centers in the United States with the scientific, technical and clinical capabilities to identify new tumor targets and then develop and infuse novel CAR-T immunotherapy. CAR- T is manufactured at UNC, making the turnaround time as low as 24 hours compared to other centers using commercial CAR-T where the turnaround time between decision to pursue CAR-T and first treatment can be up to one month.
UNC Lineberger opened its advanced cellular therapeutics facility in 2015. The facility, which is certified to use Current Good Manufacturing Practices as set by the FDA, is located approximately five miles off campus. In this facility, cellular therapy products are generated and expanded for patients receiving adoptive cell therapy for the treatment of cancer.
For more information on brain tumor research at UNC Medical Center, please contact:
Dominique Higgins, MD, PhD
Neurosurgical Oncologist
Assistant Professor, UNC Department of Neurosurgery
Email: higginsd@unc.edu
Phone: 984-974-4175
Dr. Soma Sengupta Brings Brain Tumor Clinical and Research Expertise to UNC Health
Researchers Leverage Cell Self-Destruction to Treat Brain Tumors
Neurosurgical Oncologist Dr. Dominique Higgins to Receive NRCDP Award to Fund Brain Tumor Research
Phase I Clinical Trial Using CAR-T for Glioblastoma to Begin at UNC Medical Center


Soma Sengupta, MD, PhD, MBA, FRCP, FAAN, FANA
Division Chief, Neuro-oncology
Vice Chair, Neurology.
Professor


Daniel Pomeranz Krummel, PhD
Professor


Vice Chair of Education, Clinical Skills Lab
Division Chief, Cerebrovascular & Skull Base Surgery
Professor


Diversity, Equity, & Inclusion Coordinator
Assistant Professor


Assistant Professor of Neurology and Neurosurgery