UNC Health is now enrolling patients as part of a multicenter clinical trial for newly diagnosed glioblastoma (GBM) patients. The phase 2b clinical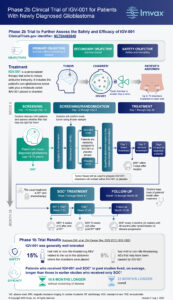 study administers a combination immunotherapy developed by biotech company, Imvax.
study administers a combination immunotherapy developed by biotech company, Imvax.
UNC Health is one of multiple academic health care centers across the country participating in the randomized trial that aims to enroll 93 patients with newly diagnosed GBM brain tumors. The purpose of the study is to assess the safety and efficacy of newly diagnosed GBM patients treated with IGV-001.
The multicenter trial is a randomized, double-blind, placebo-controlled Phase 2b study. After surgical resection of the tumor at UNC Health, participants in the trial will be implanted for approximately 48 hours with small biodiffusion chambers containing either IGV-001, which is designed to induce a broad and durable immune response against tumors, or placebo. Six weeks after treatment, all patients will then proceed with standard-of-care treatment, adjuvant chemotherapy and radiotherapy, at UNC Health.
In addition to UNC Health’s main campus in Chapel Hill, UNC Health will add 14 satellite sites within the state of North Carolina to allow patients from far away be able to participate in the study. Patients will still come for the protocol weekly visits at UNC Health’s main location in Chapel Hill, NC.
Dr. Soma Sengupta, Division Chief of Neuro-Oncology at UNC Health is the principal investigator of the study at UNC Health. Dr. Sengupta is a physician-scientist, clinical trialist specializing in brain tumor treatment and research with over 90 publications on clinical trials, lab-based research, healthcare policy and case reports. “UNC Health is the only site for this unique trial in North Carolina and in the south, making it a unique treatment option for newly diagnosed GBM patients,” said Dr. Sengupta.
Both Dr. Dominique Higgins and Dr. Carlos David, neurosurgeons at UNC Health specializing in brain tumor treatment, will be involved in the trial. Dr. Higgins and Dr. David are members of the multidisciplinary neuro-oncology clinic at UNC Health. Dr. David’s range of expertise includes all skull base and complex brain tumors and endoscopic skull base surgery. Dr. Higgins is a neurosurgical oncologist whose research and clinical focus is on the treatment of malignant brain tumors, including glioblastoma.
GBM is the most common and aggressive primary brain tumor. Patients diagnosed with GBM have a five-year survival rate of only 6.8%. Despite the aggressive nature of these tumors, the standard of care remains unchanged since 2005. Brain tumor research at UNC Health has a robust brain tumor research program and is currently conducting multiple clinical trials for GBM patients.
Imvax, Inc. is a clinical-stage biotechnology company with a unique platform technology, Goldspire™, focused on delivering personalized, whole tumor-derived immunotherapies across a range of solid tumors. The primary endpoint of the trial is progression-free survival and key secondary endpoints include overall survival and safety in GBM patients. Prior Phase 1b results were promising and showed that IGV-001 was safe and well tolerated.
If you are interested in enrolling in this study at UNC Health, please email our neuro-oncology clinical research coordinator Camisha Johnson at Camisha_Johnson@med.unc.edu or call her at (919)-445-4847 to discuss potential enrollment.
Resources:
Imvax Phase 2b Clinical Trial Fact Sheet
Written by: Makenzie Hardy, Marketing Coordinator, UNC Health Department of Neurosurgery